IVD
Microbiology-Aiming higher

In the past decade, clinical microbiology laboratories have undergone important changes with the introduction of molecular biology techniques and laboratory automation. In the future, there will be a need for more rapid diagnoses, increased standardization of testing and greater adaptability to cope with new threats from infectious microorganisms.
Laboratories had been practicing clinical microbiology the same way for decades, however over the last several years things have started to change; which is necessary as microbiology faces big challenges. The promise of rapid diagnostic technology is both inspiring and daunting, providing quality results faster than ever. However, new tests require lab personnel to both create and change infrastructure as well as strategically address problems that did not previously exist.
 Continued advances in technology to detect infectious disease will continue to fuel growth in the microbiology market. Multiple companies are developing new tests and platforms based on a range of technologies including PCR and other NAAT-based molecular tests, improvements in rapid immunoassays, mass spectrometry, next generation sequencing, and rapid phenotypic tests to determine antibiotic susceptibility. This is a promising field that is poised for rapid growth.
Continued advances in technology to detect infectious disease will continue to fuel growth in the microbiology market. Multiple companies are developing new tests and platforms based on a range of technologies including PCR and other NAAT-based molecular tests, improvements in rapid immunoassays, mass spectrometry, next generation sequencing, and rapid phenotypic tests to determine antibiotic susceptibility. This is a promising field that is poised for rapid growth.
The propelling factors for growth of the clinical microbiology market include technological advancements in microbiology testing, rising incidences of infectious diseases and outbreak of epidemics, growing healthcare expenditure, and rising private-public funding for research on infectious diseases.
Indian market dynamics
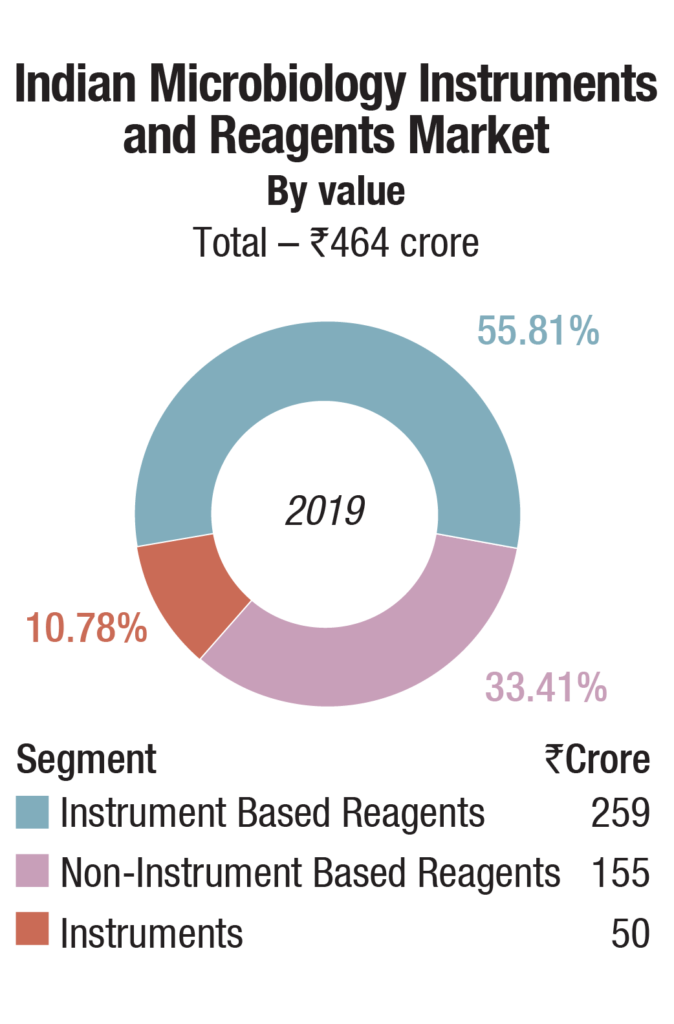
The Indian market for microbiology instruments and reagents in 2019 is estimated at Rs 464 crore. bioMérieux is a clear leader in the instruments and instruments based reagents segments, and BD is in the second place, with a combined share of about 75 percent. The other players are Beckman Coulter, Thermo Fisher, and Trivitron.
The non-instruments based reagents has many players including Hi-Media, BD India, Thermo Fisher, Bio-Rad, Microxpress, bioMérieux, Beckman Coulter, Merck Millipore, and Titan Biotech.
Indian Microbiology Instruments and reagents Market |
|
|---|---|
Mangor Players – 2019 |
|
| Segment | Vendors |
| Automated instruments market | bioMérieux, BD India, Beckman Coulter, Thermo Fisher, and Trivitron |
| Instruments-based reagents market | bioMérieux, BD India, Beckman Coulter, and Thermo Fisher |
| Non instruments-based reagents market | Hi-Media, BD India, Thermo Fisher, Bio-Rad, and Microxpress, bioMérieux, Beckman Coulter, Merck Millipore, and Titan Biotech |
| ADi Media Research | |
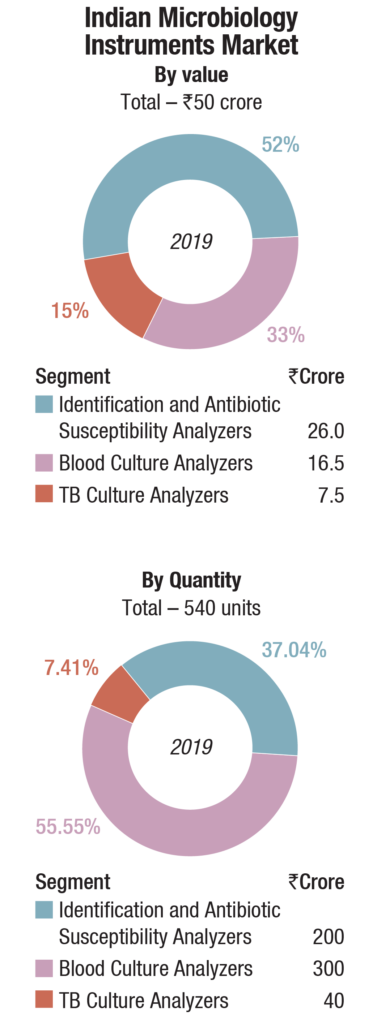
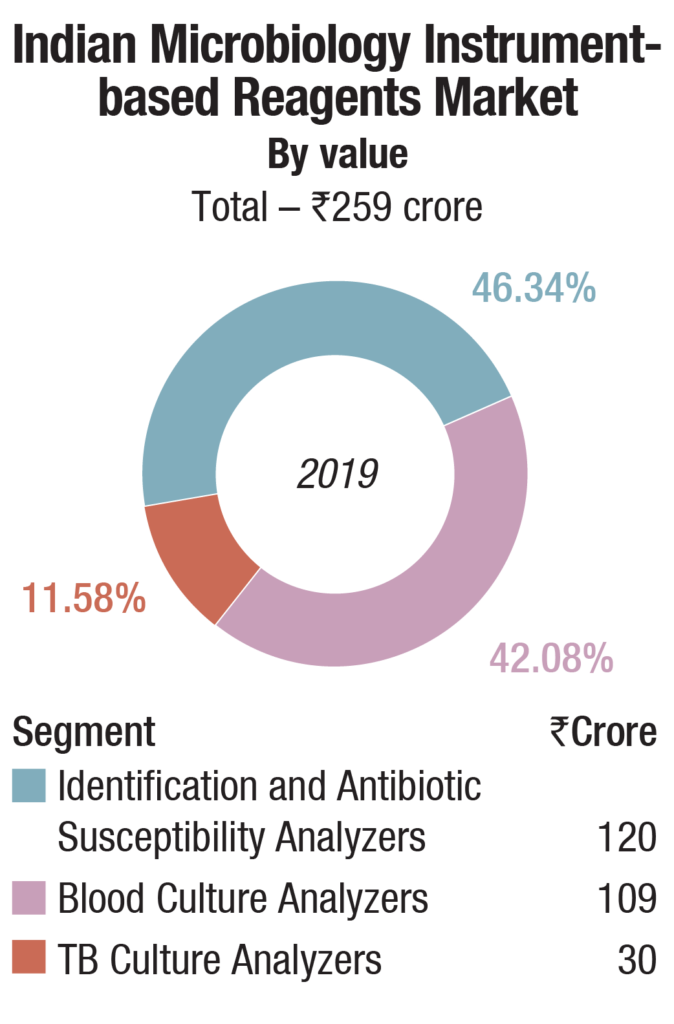
The instruments-based reagents are estimated as Rs 259 crore in 2019, a 55.8 percent share of the total market, non-instruments reagents at Rs 155 crore, at a 33.4 percent share, each having increased by 10 percent over 2018. The balance contribution was made by instruments, the market size estimated at 540 instruments, valued at Rs 50 crore, largely constituting of identification and antibiotic susceptibility analyzers and blood culture analyzers.
Automation continues to be a challenge, particularly in major clinical laboratory segments as chemistry and hematology. The complexity of developing automation suitable for microbiology tests is one of the major culprits. As the government has advised to upscale the microbiology labs at district level, this is a great opportunity for clinical microbiologists in this crisis. Over the next few years, microbiology labs might see a significant transformation from discrete manual processes to fully automated systems, which includes pre-analytical phase of processing, gram staining, and culture, including automated blood culture systems, identification, and sensitivity of microorganisms.
With high prevalence of infectious diseases, development of antimicrobial resistance, aging of population resulting in rising incidences of cardiac and other diseases, and rapidly increasing awareness about disease diagnosis and prevention. Point-of-care clinical microbiology also helps in real-time management of patients presenting with infectious-disease emergency (viruses/bacteria/fungi).
 The pandemic has played havoc, massively impacting functioning and revenues of diagnostic industry. The segment has been on a sharp decline since mid-March. During the lockdown, walk-ins (B2C business) had been significantly affected. Also, logistical challenges to move samples around, with the lockdown of interstate and intrastate movement, stopping of OPD services and elective surgeries at hospitals and clinics, corporates working from home, etc., the B2B business was also equally affected. It is anticipated that the six-month period, March- August will be quite a write-off, some activity has started picking up since September.
The pandemic has played havoc, massively impacting functioning and revenues of diagnostic industry. The segment has been on a sharp decline since mid-March. During the lockdown, walk-ins (B2C business) had been significantly affected. Also, logistical challenges to move samples around, with the lockdown of interstate and intrastate movement, stopping of OPD services and elective surgeries at hospitals and clinics, corporates working from home, etc., the B2B business was also equally affected. It is anticipated that the six-month period, March- August will be quite a write-off, some activity has started picking up since September.
New molecular methods like target amplification by different PCRs, gene sequencing, pyrosequencing, reverse hybridization, mass spectrometry, and microarray analysis help in fast and accurate identification of the causative pathogens (including viruses) as well as detection of associated resistance markers. MALDI-TOF MS used for the detection of proteins (fatty acids, metabolites of microorganisms, and oligo/polysaccharides) and DNA molecules finds application in clinical microbiology laboratory as an alternative to traditional identification systems and ELISAs and is gaining popularity in India. Point-of-care clinical microbiology also helps in real-time management of patients presenting with infectious-disease emergency (viruses/bacteria/fungi). Indian healthcare industry is a potential market in microbiology. The high prevalence of infectious diseases, development of antimicrobial resistance, and aging of population gives rise to incidences of cardiac and other diseases, and awareness on disease diagnosis and prevention.
Global market
The global clinical microbiology market is expected to reach USD 4.95 billion by 2023, growing at a CAGR of 6.4 percent, estimates MarketsandMarkets. The technological advancements in disease diagnostics, rising incidence of infectious diseases and growing outbreak of epidemics, and increased funding and public-private investments in the field of disease diagnosis are the key factors driving the growth of this market.
Reagents accounted for the largest revenue share in the global clinical microbiology market. The segment includes products such as solutions, primers, master mixes, and kits used in various diagnostic assays. Reagents currently captures the largest revenue share, with more than 60 percent attributed to repeat purchase driven by need for new set of reagents for new tests. With the advent of COVID-19 in the recent months, the segment growth has boosted enormously.
The respiratory disease segment is estimated to witness the highest growth during 2020-2023, primarily with the large patient population suffering from respiratory diseases (coupled with the growing exposure to key risk factors such as pollution) and the increasing number of epidemic outbreaks of respiratory infections.
 The global clinical microbiology market is dominated by North America. However, the Asia Pacific region is expected to witness the fastest growth during the forecast period owing to the government efforts to increase awareness related to genome-based infectious disease diagnosis; rising healthcare expenditure; increasing number of hospitals and clinical diagnostic laboratories in India and China; expanding research base across India, China, and Japan; and the increasing incidence of infectious diseases.
The global clinical microbiology market is dominated by North America. However, the Asia Pacific region is expected to witness the fastest growth during the forecast period owing to the government efforts to increase awareness related to genome-based infectious disease diagnosis; rising healthcare expenditure; increasing number of hospitals and clinical diagnostic laboratories in India and China; expanding research base across India, China, and Japan; and the increasing incidence of infectious diseases.
Over the last decade, the clinical diagnostics industry has witnessed significant changes with respect to the approaches utilized for the detection of pathogenic infections like bacteria, virus, and fungi, as well as for the diagnosis and treatment of infectious diseases such as HIV, tuberculosis, hepatitis, and influenza. The traditional empirical approach involved laboratory culture of the patient sample followed by a microscopic examination to identify pathogen or determine microbial load. With recent advances in genomics and proteomics, microbial identification and quantification methodologies have evolved rapidly. Several emerging molecular diagnostic techniques are increasingly being used for the early detection as well as effective treatment of pathogen-borne diseases.
Clinical microbiology testing procedures are not adequately reimbursed across a number of countries like India, China, and other emerging countries in the Middle East and Africa, which have a high target patient population base. This is a major factor that limits the preference for clinical microbiology products among patients and healthcare professionals. This directly affects the adoption of premium-priced clinical microbiology products, thereby negatively affecting their market growth.
Key players operating in the clinical microbiology instruments and reagents include bioMérieux, Danaher Corporation, Becton, Dickinson and Company, Abbott Laboratories, Bio-Rad Laboratories, F. Hoffmann-La Roche, Bruker Corporation, Hologic, Qiagen, Thermo Fisher Scientific, Agilent Technologies, Merck, Shimadzu Corporation, 3M Company, and Neogen Corporation.
Buyer’s perspective
All clinical microbiologists agree that providing accurate results quickly is essential to providing the best care for patients, but implementation of new technologies is a decision to carefully consider. The microbiology lab staff must discuss all aspects of implementation (not limited to cost) to ensure that the benefits outweigh the risks.
What does this conversation potentially look like? What are the elements worth considering before bringing rapid diagnostic technology to the micro lab?
Second Opinion
MALDI-TOF a revolution in clinical microbial identification
Dr Mamta Kumari
Chief Microbiologist and DGM,
Clinical Reference Lab SRL Ltd, Gurgaon
The field of clinical microbiology nowadays is rapidly evolving because of several recent technological advances. From walk-away multiplex PCR assays to total laboratory automation, microbiologists are able to produce faster results of higher quality than ever before. Perhaps the most revolutionary advancement over the last decade has been the application of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) for the identification of organism. Not only is MALDI-TOF MS organism identification more accurate than growth-based systems, but identifications are produced more rapidly and at significantly lower cost. This technology can identify gram-positive, gram-negative, aerobic, anaerobic bacteria as well as mycobacteria, yeast, and molds, typically at the species level, with good accuracy and often better than traditional methods when compared to sequencing.
As the technology has evolved, the expansion of the databases containing spectra of known organisms has allowed the identification of species with similar phenotypic, genotypic, and biochemical properties that was not previously possible. This has resulted in improvements in clinical care including the diagnosis of infections caused by relatively rare species and decreasing the time to diagnosis. Overtime, the platforms have gotten progressively better, with significant improvements in the software, interpretive rules, and databases.
MALDI-TOF MS can be used for various purposes like, microbial identification, strain typing, epidemiological studies, detection of biological warfare agents, detection of water- and food-borne pathogens, detection of antibiotic resistance and detection of blood and urinary tract pathogens etc.
The provision for integrating in-built databases in the public databases of MALDI, as well as development of inexpensive and user friendly software for comparison and analyses would further increase the credibility of MALDI-TOF in future.
Like any other technology, it also has some limitations like inability to discriminate between very closely related species that can be due to inherent similarity of the organisms among themselves or in case of mixed infections. These errors are relatively low frequency and can be overcome by a supplemental testing.
In conclusion, the adoption of MALDI-TOF in clinical microbiology has revolutionized the infectious disease diagnosis and clinical care due to timely and accurate identification of microorganisms. This has further lead to appropriate therapy resulting in reduced hospital stay of the patients.
Justifying the implementation of modern technology involves several components in addition to the cost. Every lab is different and what makes technology work for one lab may not work for another. The most important items to take into account when considering the implementation of rapid diagnostics include:
Infrastructure. Staffing, the existence of a stewardship program, the electronic medical record system and specimen volume are all critical pillars of support for rapid diagnostic technology.
Choosing the right test. Since cost is a major factor, picking the test that will offer the most bang for buck is important. Consider the patient mix and what relevant stakeholders need to get from the test.
Considering how the test affects other organizations. Consider if the test will allow continuing to support public health efforts. Make a plan to implement factors that continue to support the clinical lab and public health lab relationship.
Re-structuring classic methods. Figure out how to fit traditional microbiological methods, like culture, into the new molecular landscape, which will be imperative for success.
Often, the implementation of molecular technology does not mean that the older microbiological methods go away. Even though the use of molecular technology provides accurate organism identification within a few hours, bacterial culture is still required to obtain complete antimicrobial susceptibility information. At this time, growth of an organism on media is required to determine a susceptibility result, which plays an important role dosing of some antibiotics. Additionally, antimicrobial susceptibilities are key to building antibiograms, which aid clinicians in assessing local susceptibility rates. The lab should be prepared to consider the cost and staffing needs of continuing to perform culture when implementing rapid methods.
While the respiratory panel offers identification of 21 respiratory pathogens, only a few of those can be treated, so why test for the ones that cannot be treated? Are clinicians over-using this technology? When is it better to implement a multiplex PCR when a major pathogen (that can be treated) can be detected using a single PCR?
Hospital volume. If the testing volume is low, a multiplex panel may not be appropriate and may offer little clinical value. Choosing a PCR that targets a disease with the most clinical impact (influenza) may be the best choice.
Patient population. If the patient mix includes complex cases and patients with immunosuppression, testing for pathogens other than the ones that can be treated can still be beneficial. It is important to remember the role infection control plays, as patients may need to be isolated based on certain test results.
Laboratory setup. Based on staffing and space, it is important to consider if it would make more sense to batch specimens and run them all at once or run them one at a time as they are received.
Although the benefits of diagnostics in the clinical setting are clear, the impact on public health laboratories should be taken into consideration before implementation. Several common molecular platforms have the potential to put the public health laboratory process at risk of falling behind.
The cost of a rapid diagnostic platform cannot be the only factor to consider, it might be the one that prevents from bringing the instrument in-house entirely. One of the biggest barriers to rapid diagnostic test implementation is cost. Although companies claim that cost-savings from the use of their technologies are enough to cover capital expenditure and reagent cost, buyers will likely have to justify these savings within their institution in order to obtain the equipment. It is important to also consider the cost associated with strengthening of the infrastructure when justifying the implementation of new rapid diagnostic technology.
Rapid diagnostics are only effective if implemented in a setting with appropriate infrastructure and used as intended. Implementing expensive technology without working through these considerations may leave the lab like a car without gas: running on empty.
Clinical microbiology in low resource settings
The field of clinical microbiology faces increased pressures in low and middle-income countries (LMICs) where the burden of infection is highest and health systems appear least able to respond to pressures, such as antimicrobial resistance. The current gaps in knowledge relating to detection, characterization, effective treatment, and follow-up constrain national governments and international organizations in their efforts to detect evolving trends and emerging threats.
Good quality clinical microbiology, implemented at a population level through well-functioning reference laboratory networks is necessary for effective antimicrobial resistance surveillance and control for example, but low-resource settings face infrastructural, technical, and human resource challenges toward such an implementation. As is true for many topics, hospitals and communities in different LMIC settings have unique challenges and methods for overcoming those challenges fully or partially. The regional and national variations of clinical microbiology implementation in LMICs limit understanding and the extent to which clinical microbiology results can inform national and international health policies. An important component and common limitation of health systems in LMICs are the human resources to do work, across all cadres of staff, including clinical, laboratory, managerial, policymaking, data analysis, and project management groups.
Microbiological expertise is particularly limited in the generation, sharing, systematic analysis, and dissemination of data in low-resource settings. Numerous agencies and initiatives are working to support the development of clinical microbiology capacity. However, the routine generation of a predictable, sustainable flow of reliable data will take time to establish and deliver clinical and public health impact. An integrated model, combining clinical, laboratory, and demographic information remains perhaps a distant aspiration. As such the need to share knowledge and experiences is an effective approach to remove communication barriers and contribute to reducing the inequities in understanding disease burden worldwide.
The expectation for clinical microbiology laboratories to be technology-ready and able to swiftly incorporate new technological solutions is currently difficult to materialize. New technologies like next-generation sequencing diagnostics do have the potential to increase the surveillance granularity of microbial pathogens and eventually widen the opportunities for more effective healthcare implementation. They have also proven workable when operated in isolation. However, there are a number of foundational needs that still need to be addressed in LMIC settings. For example, the standardization or at least harmonization of protocols for existing methodologies across different geographies—even within the same country—remains a critical first step.
Dr Rameshwari Bithu
Sr Professor Dept. of Microbiology
SMS Medical College & Hospital, Jaipur
“Looking into the recent scenario of emerging and re-emerging infections and threat of biological welfare to humanity, microbiology has become an important branch of science helping in early diagnosis of various infecting agents, prompt management of cases, and saving life. Automation in the field of microbiology plays an important role. The easy procurement process and quality after-sale-services of machines/equipment is essential for smooth functioning of laboratory, in the interest of patient
In India, public health facilities plays an important role in providing quality health services to large section of society. Procurement of quality equipment and reagents is difficult in government sector health services due to financial constraints (firms quoting lower prices and tender are selected irrespective of the quality). Firms supplying microbiology machines/instruments and reagents should have superior facilities for demonstration of their product by learned technical support, as such procurement and budgets in the government setup is decided by the administrative wing of the institutions”.
Moving forward
The environment of the clinical microbiology laboratory is rapidly changing. Testing methodologies based upon organism growth on an array of liquid and solid media are being replaced by newer methods that enhance the rate of organism identification as well as increase the sensitivity and specificity by which identification occurs. A majority of these new techniques are based upon nucleic hybridization and polymerase-chain reaction technology and range from identification of single-organisms or organism families to multiplexed syndromic panels, which can concurrently examine for the presence of numerous suspect organisms based upon the symptoms exhibited by the patient.
In addition, the clinical microbiology laboratory now has access to a level of automation thus far only seen in the chemistry and hematology sections of the clinical laboratory. These transitions have been repeatedly shown to enhance the level of patient care when properly implemented into the laboratory workflow. Conversely, with the rapid encroachment of these new technologies comes potential downfalls, including cost and challenges with training laboratory staff.
Collectively, the clinical microbiology laboratory is coming into a new era of technology and patient care that will bring about dramatic changes to conventional testing and organism identification.











