Microbiology Instruments and Reagents
Changing diagnostic paradigms for microbiology
Automation in microbiology is not simply automating the traditional practices of processing specimens and incubating cultures – that would not be transformative. Recently described as a paradigm shift in clinical microbiology, it represents the beginning of the future.
Clinical microbiology has entered a new era of diagnostic testing, in which molecular tests have transcended the confines of the basic science research laboratory, and become important tools in the clinical laboratory. Traditional and cumbersome polymerase chain reaction (PCR) methods have given way to simpler, faster, and less costly techniques that can be used by smaller clinical laboratories that lack extensive training in molecular methods. The past decade has seen a surge in the development of various molecular diagnostics to rapidly identify or characterize infectious microorganisms, with each test designed to improve sensitivity, specificity, and/or turnaround times of conventional methods.
For the past several decades, clinical laboratories have been asked to do more with less, and diagnostic microbiology has been no exception. Labs are asked to accurately detect antibiotic-resistant microorganisms, screen patients for hospital-acquired infections, provide more and more rapid results to assure decreased patient length of stay, rapidly test hundreds to thousands of patients daily for severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2), and more. All of this is in the face of decreasing laboratory resources. Labs are receiving less and less reimbursement for the testing they perform, undergoing laboratory consolidations (often without accompanying resources for the increased workload), receiving unfunded legislative mandates, undergoing budget cuts, and facing significant decreases in workforce, all of which make it very challenging to meet these expectations.
Laboratory consolidation brings challenges. Testing is potentially moved away from physicians at a time when the interpretation of sophisticated tests requires active collaboration with the microbiology staff. Also, concentration of diagnostic procedures in large centralized laboratories poses increased safety risks for the technical staff at a time when infections with antimicrobial-resistant organisms and other communicable pathogens are increasing. One can accept these changes as inevitable problems, or one can view them from another perspective.
Laboratory automation came to chemistry, hematology, and immunology decades ago and more recently it has reached microbiology.
Automation in microbiology is not simply automating the traditional practices of processing specimens and incubating cultures – that would not be transformative. Automation should be viewed as an all-encompassing approach to processing samples and communicating information that enable laboratory technologists to perform skilled tasks in ways not historically possible. This is why automation was recently described as a paradigm shift in clinical microbiology, representing the beginning of the future.
Automation in microbiology enables laboratories to do much more than eliminate the manual repetitive practices and inefficiencies of the past – the laboratory noise. Realizing the full value of automation, on the other hand, necessitates a thorough re-evaluation of the entire laboratory workflow.
The Indian market for microbiology instruments and reagents in 2020 is estimated at ₹350 crore.
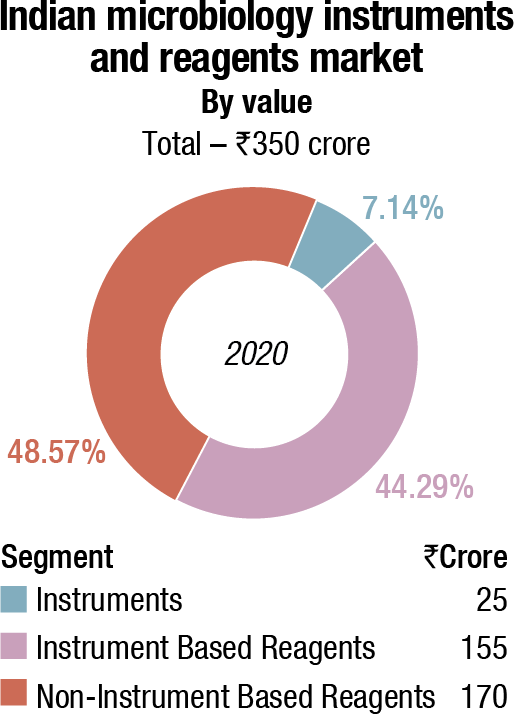
The instruments-based segment constitutes 51.43 percent of the market, with the analyzers at ₹25 crore and the reagents at ₹155 crore. The non-instruments-based reagents contribute the balance 48.57 percent to the market at ₹170 crore.
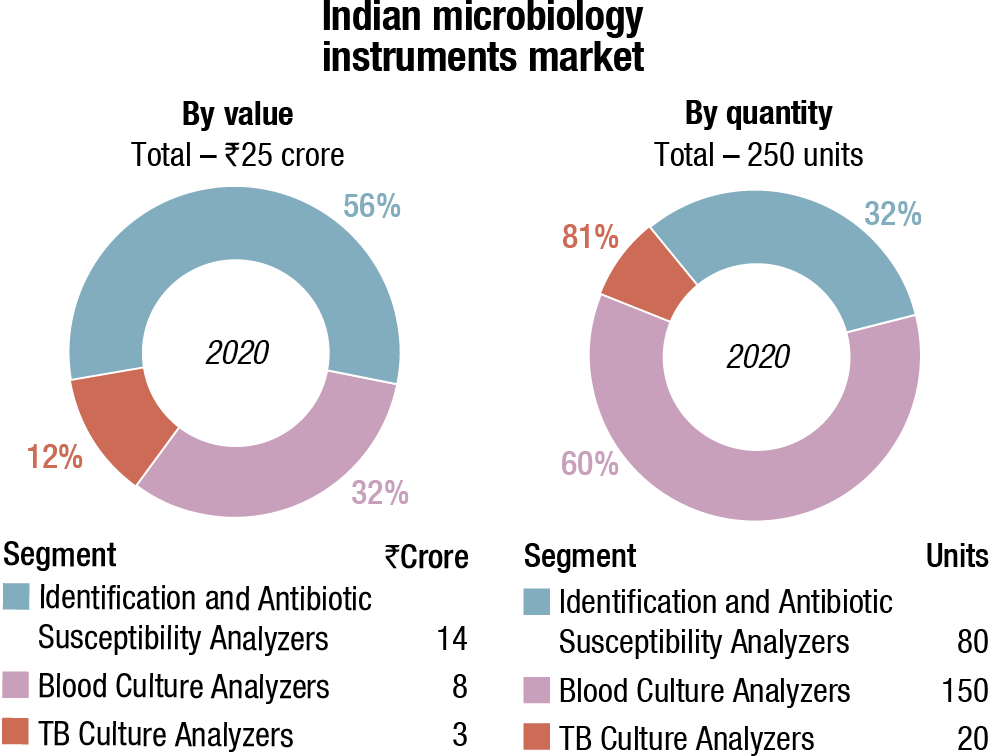
bioMérieux is a clear leader in the instruments and instruments-based reagents segments, and BD is in the second place.
| Microbiology instruments & reagents market | ||
| Major players – 2020 | ||
| Tier I | Tier II | Others |
| bioMérieux | BD | Thermo Fisher & Trivitron |
| bioMérieux & BD | Rapid & DL Biotech, China | – |
| BD | Midget & bioMérieux | – |
| ADI Media Research | ||
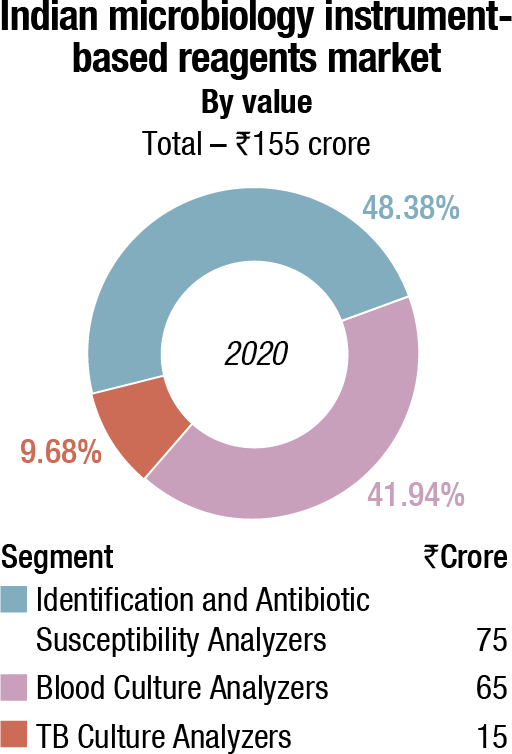
In the non-instruments-based reagents segment, Hi-Media dominates with an estimated 58-percent share. This includes ready-to-use plates, antibiotic disks, MICs, etc. Titan Biotech (Microexpress) and Tulip are the other aggressive players.
| Microbiology non-instruments & reagents market | ||
| Major players – 2020 | ||
| Tier I | Tier II | Others |
| HiMedia | Tulip & Titan (Microexpress) | Thermo Scientific (Oxoid), bioMérieux, BD India, Bio-Rad, Beckman Coulter (Siemens), Merck Millipore. Additionally, regional players have a combined 14% market share |
| ADI Media Research | ||
In 2020, the COVID-19 pandemic raged through the country, and impacted microbiology testing that had to be on the backburner. The six-month period from March to September was an almost complete write-off; regular testing only started in October 2020.
When an increasing number of patients with COVID-19-associated mucormycosis were reported, then the microbiology testing received some impetus. Specimens to test the disease vary according to the type of infection and clinical presentations. Most commonly used samples include skin scrapings from cutaneous lesions, nasal discharges, scrapings and aspirates from sinuses in patients with rhinocerebral lesions, bronchoalevolar lavages and needle biopsies from pulmonary lesions, and biopsy tissue from patients with gastrointestinal and/or disseminated disease. From pre-COVID era, prevalence of mucormycosis was at least 70 times higher in India as compared to other countries.
Growth resumed for blood-culture reagents and culture media during the last quarter too, but overall performance remained impacted by the decline in demand in other products and in equipment sales.
The market growth is slated to be augmented by outbreaks of pandemics including COVID-19, Ebola, and Zika virus, leaving a large number of infected people.
Amid the COVID-19 crisis, the global market for clinical microbiology instruments and reagents estimated at USD 4 billion in the year 2020, is projected to reach a revised size of USD 5.8 billion by 2026, growing at a CAGR of 6.4 percent, estimates Global Industry Analysts. Instruments segment is projected to record 5.3 percent CAGR and reach USD 2 billion by the end of 2026. After a thorough analysis of the business implications of the pandemic and its induced economic crisis, growth in the reagents segment is readjusted to a revised 7.1 percent CAGR for the next seven-year period. Growth in the reagents segment is due to increasing ageing population that is vulnerable to microbial infections and chronic conditions, such as bacterial pneumonia, urinary tract infections, gastrointestinal issues, renal diseases, and cancer. The market growth is slated to be augmented by outbreaks of pandemics including COVID-19, Ebola, and Zika virus, leaving a large number of infected people.
The clinical microbiology instruments and reagents market in the US is estimated at USD 1.6 billion in the year 2021. China, the world’s second largest economy, is forecast to reach a projected market size of USD 284.1 million by the year 2026, trailing a CAGR of 7.7 percent. Among the other noteworthy geographic markets are Japan and Canada, each forecast to grow at 5.2 percent and 6.3 percent respectively. Within Europe, Germany is forecast to grow at approximately 6.3 percent CAGR. The US and Europe constitute major markets for automated and rapid microbiological tests. Emerging nations, such as China, India, and Brazil, present significant opportunities due to their constantly growing healthcare infrastructure. The Asian market presents substantial growth opportunities owing to rising prevalence of various pathogenic diseases, requirement to accelerate microbiological testing, and discovery of adapting and mutating bacterium.
Clinical microbiology holds a prominent role in the field of research and medicine, and supports the fight against numerous infectious diseases along with antimicrobial resistance. High prevalence of infectious diseases and sexually transmitted diseases will continue to drive the future of automated and rapid tests, as the need for quicker, higher volume, and more accurate methods of testing gains significance. Emerging technologies and robust processes have thus transcended microbiology testing to the next level. Rising healthcare expenditure, growing need to speed up microbiological testing, scarcity of time, need for rapid QA-QC procedures, and growing imposition of regulations that regulate various production processes are factors that have catalyzed this transformation. The uptake of microbiology is further favored by technological advancements including automated instruments to identify pathogens across laboratories.
Improving healthcare infrastructure across emerging countries. Emerging markets are expected to offer significant growth opportunities to clinical microbiology product manufacturers and distributors in coming years. This can be attributed to the growing prevalence of infectious diseases, such as HIV, tuberculosis, influenza, and malaria as well as increasing R&D initiatives to develop innovative genomic techniques for efficient disease diagnosis in developing nations.
The growth in these markets is further supported by improvements in healthcare infrastructure, growing healthcare expenditure, and increasing availability and affordability of low-cost clinical microbiology products. In line with the ongoing trend, several government initiatives have been undertaken across emerging markets (particularly in China and India) to strengthen and expand the healthcare infrastructure.
Limited reimbursement policies for microbiology testing procedures. Clinical microbiology testing procedures are not adequately reimbursed across several countries, such as India, China, and other emerging countries in the Middle-East and Africa, which have a high target patient population base. This is a major factor that limits the preference for clinical microbiology products among patients and healthcare professionals. This directly affects the adoption of premium-priced clinical microbiology products, thereby negatively affecting their market growth. Laboratory tests performed for in-patients are reimbursed along with diagnosis-related group payment only, whereas reimbursements for out-patient laboratory tests are made locally. The lack of national standardization for reimbursements results in significant variation at the regional level. The adoption of microbial tests in the US is further restricted due to limited coverage for operational costs for these test procedures, restricted research use of only diagnostic products, and the slow inclusion of newly developed microbial test procedures in CPT codes for medical reimbursements.
Operational barriers. Clinical laboratories across major markets are still evolving; technicians face operational challenges in ensuring effective sample procurement, storage, and transportation, especially while adopting novel technologies, such as NGS and lab-on-a-chip PCR devices. Laboratory space also needs to be reconfigured to meet the requirements of conducting specific diagnostic tests used for pathogen detection as a means of avoiding cross-contamination and ensuring efficient time management. This results in considerable cost escalation to maintain and operate advanced rapid microbiology tests, particularly those capable of handling a single-sample type. Furthermore, due to the rapid mutation of microbes and the increasing outbreak of epidemics, clinical laboratories need to adopt innovative technologies capable of rapid sample diagnosis.
Some of the leading competitors are Abbott Laboratories, Thermo Fisher Scientific, Agilent Technologies, Merck KGaA, bioMerieux S.A., Cepheid Inc., Danaher Corporation, Bruker Corporation, Becton Dickinson & Company, Hologic Inc., Roche Diagnostics, and Alere Inc. Clinical microbiology companies have announced mergers and acquisitions, partnerships and collaborations, and new product development to expand their position in the clinical microbiology industry. Major players are also moving into new regions or advanced technologies for gaining a competitive advantage.
Fully automated diagnostics pipeline is a seducing idea and first automated microbiology laboratories have started to be implemented world-wide. In parallel, machine learning (ML), a branch of artificial intelligence (AI), has gained a foothold in many fields of clinical medicine. Industry actually has ML-driven tools that can make diagnosis and help clinicians in decision-making challenges, such as the choice for a given treatment, and even empower the patients themselves to manage their healthcare. The innovative aspect of ML is that it is not a ruled-based system; ML algorithms can learn from input data and automatically make predictions or decisions.
With next-generation sequencing (NGS) techniques, one can gain information about pathogens, analyzing millions of small fragments coming from their genomes and even gain insights on microbiota composition, including not-yet cultured or uncultivable organisms.
Can automation, together with new technologies, make a difference from conventional clinical microbiology tests that often require a significant amount of manual work?
What impact will such advancements have in clinical routine in terms of sample-to-result timing, taking into account that it usually takes between 24 and 48 h to obtain results in current routine laboratories? What will such new technologies imply in terms of resources and management? Lastly, can experts understand and interpret multimodal large-volume data resulting from these new technologies?
The implementation of new technologies, like automation, ML, and NGS brings several issues. Automation of a clinical microbiology laboratory is challenging until it can reach all the steps, like opening all routinely used sample containers, relying on validated incubation times and standardized antibiotic susceptibility testing.
Standardization and validation of the pre-analytical, analytical, and post-analytical procedures are needed before the automated system is fully applicable to routine analyses. In this respect, tasks of the automated pipeline could be segmented and sequentially validated allowing also a better management of personnel training and implementation of instruments in the hospital routine daily life. Importantly, an appropriate IT system should be put in place to ensure a correct information exchange with the automated system, e.g., for the protocol of the microbiological tests/tasks to perform.
 Dr Mamta Kumari
Dr Mamta Kumari
MBBS MD (Microbiology),
SRL Clinical Reference Lab, Gurgaon
There have been sweeping advances in the deployment of molecular, genome sequencing-based, and mass spectrometry-driven detection (MALDI–TOF), identification, and characterization assays in the field of clinical microbiology. Automations in the laboratory and integration of information systems for data management are also being established. Although there was high enthusiasm for these approaches initially, we have entered a phase in which we are navigating the challenges and complexities associated with them, rather than just the healthcare benefits. Keeping this in mind, the ongoing process of clinical laboratory consolidation seems like the right step forward as it not only covers large geographical regions but also represents an opportunity for the efficient and economical introduction of new lab technologies and improvements in translational research and development.
As data increases in bulk and complexity, further technological advancements will change the face of clinical microbiology laboratories. But appropriate implementations in real clinical setup will ensure that the results are faster and cleaner than conventional workflows. A large, structured repository of bio-banked biological materials from networked libraries will create numerous opportunities for clinical research of infectious disease and in turn produce impactful healthcare benefits. Consolidation of clinical microbiology laboratories will prove to be advantageous for most aspects of service that distinct institutions already provide individually. Some of the areas that can be aided by innovative and large-scale diagnostic platforms are infectious disease detection, surveillance, and prevention with new translational research and enhanced diagnostic product and service development opportunities.
Biosafety is also an important aspect that should be carefully considered when implementing a new system to appropriately handle clinical samples with biological hazard, in order to prevent accidental infections among laboratory personnel or laboratory contaminations.
ML-driven technologies are black boxes, meaning that the processes leading from the input to the output are unknown to the user. Therefore, although ML represents a promising tool, especially in coping with large-volume complex data, the understanding of its functioning might be hard for microbiologists and clinicians who must inspect and validate the results.
Furthermore, ML-driven technologies should be examined in clinical trials in order to be safely and officially incorporated in laboratory-certified operations. Thus, whether ML approaches bring an added value to diagnostics remains to be clarified, once routine implementation can be achieved and potential benefits measured.
NGS and metagenomics are neither fully standardized, nor streamlined in a way that they can smoothly integrate a routine microbiology laboratory. Some efforts to converge toward national/international validated procedures have been undertaken.
Moreover, given the large volume of sequencing data, metagenomics can demand a lot of computing resources and can be time-consuming. NGS can detect species in terms of relative abundance to which one should find a meaningful corresponding parameter to allow comparison with culture data.
Automated systems and NGS require the availability of suitable host facilities, trained personnel, and adequate informatics infrastructure for data computation, analysis, interpretation, and storage. In the absence of such factors, small hospitals are excluded from these technological advancements.
Therefore, a reorganization of diagnostics laboratory networking is warranted. Although different models of automated clinical microbiology laboratories are currently implemented, they are all characterized by a central facility with one or more satellite laboratories. While the central facility should incorporate all the current key technologies, including automatized system and NGS, satellite laboratories serve as platforms for rapid response tests.
Particular attention should be put at data communication and sharing. One can imagine that these exchanges develop at three different levels – between personnel (clinicians, laboratory operators) belonging to the same hospital facility; between personnel from satellite and central facilities of the same hospital corporation; and between different hospitals.
Electronic health record is the systematic collection of patient information in digital machine-readable format and represents a solution to data communication and interoperability between the disparate hospitals, on condition that consistent ontology definitions are used. The findability, accessibility, interoperability, and reusability initiative principles should be considered to generate formal diagnostic concepts, and to define standard diagnostic definitions used in electronic health records. A constant curation and revision of ontologies should then be ensured, especially when new technologies are introduced in routine analyses. This is the case of genomics, where information is very often not structured in a machine-readable format where new technical terms and new types of data representation are introduced. Therefore, the constitution of a data report for genomic data, which is largely understood and accepted by the clinicians, should be evaluated.
Exchange of clinical data between infrastructures implies that patient privacy should be guaranteed at any operation level and an ad hoc security system should be used. Privacy-protecting technologies like homomorphic encryption and secure multiparty computations could ensure a protected environment where to store or locally analyze data that is without the need to electronically transfer them to another informatics environment. Implementation of secure computation, based on cryptographic protocol that covers the features of patients, has also been proposed for the analyses of microbiome.
New technological advancements are going to change the appearance of clinical microbiology routine laboratories with data increasing in volume and complexity. Yet, their implementation in real clinical settings should still prove an improvement in making processes faster and cleaner than conventional workflows. Explainability and interpretability of ML-based tools are rarely addressed, and independent validations should be carried out. A re-arrangement of local and regional diagnostics facilities is demanded to better cover the needs of management of automated laboratories.












