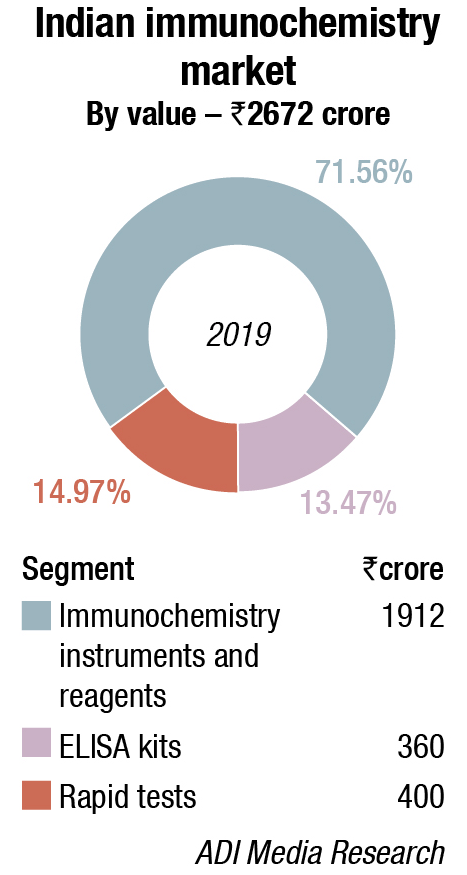
Immunochemistry Instruments and Reagents
Contrasting roles of immunoassays in diagnostics
Immunochemistry is the backbone of diagnosis and prognosis of diseases in healthcare system, and is thus the need of today for accurate and precise measurement of the sensitive parameters, and for their rapid diagnosis.
 The urgent need for accurate and rapid diagnosis of SARS-CoV-2 infection remains critical as global healthcare systems continue to operate during the course of the COVID-19 pandemic. In particular, serological and immunological testing of infected asymptomatic and symptomatic individuals, and their close contacts, is expected to be in high demand.
The urgent need for accurate and rapid diagnosis of SARS-CoV-2 infection remains critical as global healthcare systems continue to operate during the course of the COVID-19 pandemic. In particular, serological and immunological testing of infected asymptomatic and symptomatic individuals, and their close contacts, is expected to be in high demand.
A new technology for COVID-19 detection has become available that is much simpler and faster to perform than currently-recommended nucleic acid amplification tests (NAAT), like PCR. This method relies on direct detection of SARS-CoV-2 viral proteins in nasal swabs and other respiratory secretions using a lateral flow immunoassay (also called an RDT) that gives results in < 30 minutes. Though these antigen detection RDTs (Ag-RDTs) are substantially less sensitive than NAAT, they offer the possibility of rapid, inexpensive, and early detection of the most infectious COVID-19 cases in appropriate settings.
A number of immunoassays have received approval for diagnostic use for COVID-19, utilizing formats like ELISA and lateral flow.
Significant progress has been made in the development of diagnostic tests despite all the remaining questions and challenges. Ongoing global efforts are working to communicate and facilitate new diagnostic assay development and worldwide test kit delivery. To promote more accurate and faster diagnostic solutions, a number of organizations are supporting these efforts by inviting assay developers to submit their test products for independent evaluation or by providing huge investments for greater collaboration.
As similar initiatives and knowledge sharing become available, including collaborative technological advancements, it is likely that the COVID-19 diagnostic market will continue to thrive well into the future.
Market trends
Immunochemistry has witnessed extraordinary advancement in every aspect of basic and clinical research, and this is owing to technological advances, which enable quantitatively and comprehensively measuring immune response to genetic or environmental perturbations. Immunochemistry analyzers are becoming more and more popular these days as they have proved to be very effective tools to diagnose cancer, hepatitis, illegal drugs, fertility problems, infectious diseases, and many chronic diseases.
 The author is Dept of Biochemistry, IMS & SUM Hospital
The author is Dept of Biochemistry, IMS & SUM Hospital
“Alongside qRT-PCR, immunoassays are being evaluated for rapid detection and screening. Antibody to nucleocapsid protein and spike proteins are detected by using luciferase in immunoprecipitation methods. A colloidal GICA strip and a peptide-based MCLIA are used to detect SARS-CoV-2 IgM and IgG antibodies. Use of ultrabright fluorescent nanoprobe is expected to raise sensitivity by 100 times by increasing fluorescence signal to background noise for Ag detection. Another procedure to detect viral Ag at the concentration of 100fg/ml is by using a Pd nanoparticle functionalized electroactive Co-MOF for signal amplification. Sensors based on carbon or carbon polymer dots, or Fe3O4 nanozyme amplifies the signals of the photoelectrochemical immunoassay. Using Raman spectroscopy nanotags also viral particles can be effectively detected. Microfluidics are being used for salivary sample analysis for viral antigens.”
With chemiluminescence immunoassay (CLIA) systems gaining momentum; this in turn results in a decline in ELISA tests. RIA and Fluorescence Immunoassay (FIA) are age-old procedures, which over time have been replaced by ELISA. Rapid tests can reveal the test results in less than 30 minutes; hence these are preferred for screening. ELISA, on the other hand, continues to be trusted as a gold standard, owing to extremely high sensitivity, specificity, precision, and throughput.
The last two decades have seen tremendous automation in ELISA technology. From a compact bench-top micro-strip processor, ELISA processors are now available with six plates, allowing reporting of over 500 test results at one go. Automated processors are equipped with robotic probes and dedicated workstations, which drastically improve ELISA workflows.
These fully automated processors are complete walkaway systems that minimize manual errors, while improving the accuracy and turnaround time for critical tests. Usually being open systems, the limitation of ELISA kits from a single source can also be ruled out.s
The diagnostic landscape in India is largely varied, with the chain labs concentrated in the big metros, and the rural areas often devoid of even the most basic facilities for routine testing. Moreover, public hospitals in metros are often overloaded. In either of these scenarios, ELISA is often the preferred choice for being affordable and reliable.
The evolution of diagnostic and prognostic markers is significantly changing the clinical practice, and this can be demonstrated by the use of immunochemistry. The demand for immunochemistry has increased owing to overall increase in healthcare expenditure, decreasing mortality, increased awareness, and lifestyle-related diseases.
Immunochemistry is the backbone of diagnosis and prognosis of diseases in healthcare system, and is thus the need of today for accurate and precise measurement of the sensitive parameters, and for their rapid diagnosis.
Connecting mass spectrometry to chemistry and immunoassay analyzers
Going forward, more novel technologies, once reserved for specialized settings, such as molecular/virology, may increasingly migrate into the core laboratory.
Efforts are underway to connect both mass spectrometry and molecular diagnostics instruments to clinical chemistry systems. If mass spectrometry could be connected to chemistry and immunoassay analyzers on the same platform, drugs of abuse testing could be done in real time, with samples moving directly from immunoassay screening to confirmatory testing. Likewise, if molecular diagnostics instruments were to connect to core laboratories, chemistry and immunoassay testing could be combined for diagnosing and monitoring infectious diseases, with follow-up molecular confirmation using the same sample on the same track. The risk of contamination for molecular diagnostics tests has been a barrier in the past, but companies are coming up with newer ways to manage this risk.











