MB Stories
COVID-19 results in exponential growth for MDx in 2020

The Indian MDx market saw an additional ₹7000 crore sales on account of RT-PCR testing.
As the world rushes to become vaccinated against the COVID-19, the strategy turns from infection mitigation to achieving herd immunity. The pandemic and the multiple lockdowns that followed have had a huge effect on the world’s markets, butchering some industries while allowing others to boom. The medical device field has been no exception, but different sub-segments have been affected differently.
Last year saw the onset of the virus in the Western world and the lockdowns and shutdowns that followed. This meant that the field of elective surgeries saw its sales numbers tank, as everyone was staying home and surgeries were postponed. Some companies saw as much as double-digit declines in their sales segments.
These same decisions, however, led to growth in the testing market. As COVID-19 tore across the world, correctly identifying who had the virus was of paramount importance to enforce quarantines and stay-at-home orders. Polymerase chain reaction (PCR), antigen, and antibody tests skyrocketed from their original workflows to having to produce hundreds of millions, if not billions, of tests very quickly. But this explosive growth will not continue forever.
While IVD companies may still report modest growth numbers in future, as more and more of the world becomes immunized, the demand for tests will decrease until they are no longer needed.
This may lead to an unfortunate situation for the molecular diagnostics market. As the need for COVID-19 testing collapses to a fraction of its current demand, molecular diagnostics companies will be holding and maintaining facilities that can create immense supply. This mismatch between demand and supply will either lead to a collapse in prices of molecular diagnostic tests, as suppliers struggle to break even on their facilities maintenance costs, or a mass selloff or repurposing of these facilities.
Either way, the companies in the molecular diagnostics space will have to plan their next steps very carefully. They have made a lot of money in the boom of the COVID-19 pandemic, but they must be careful not to be saddled with large illiquid material assets for a market that is a fraction of its previous price.
Since the COVID-19 outbreak, India has been at the forefront of formulating COVID-19 testing protocols. With an average daily testing of more than 1.7 million in the month of August, India has tested 500 million samples across the country till date.
India has achieved the milestone of the last one hundred million tests in only 55 days. On July 21, 2021, India had tested 450 million COVID-19 samples, which reached the 500 million mark on August 18, 2021. This has been enabled by rapidly increasing testing infrastructure and capacity across the country. ICMR has been enhancing COVID-19 testing capability across the country by expanding and diversifying testing capacity by leveraging technology and facilitating innovation in affordable diagnostic kits. The testing strategy has been carefully calibrated to increase access and availability of testing.
The concerted efforts toward augmenting and diversifying testing prepared the infrastructure, which made it possible to deliver on India’s increased testing requirements in the wake of the second wave of COVID-19. COVID-19 testing capability across the country has been enhanced by leveraging technology and facilitating innovation in affordable diagnostic kits. Easy-at-home self-diagnostic kits have been developed and approved.
A specific testing platform is available, addressing general testing (RT-PCR), high-throughput testing (COBAS), testing at remotest places and PHCs (TrueNAT, CBNAAT), in containment areas (rapid antigen testing) and for large number of migrant population (pooled sample testing). The total number of diagnostic laboratories has reached 2876, of which dedicated government laboratories are 1322 and private laboratories’ number stands at 1554.
| Indian Molecular Diagnostics Instruments & Reagents Market | |
| Leading players – 2020 | |
| Segment | Some leading companies |
| Analyzers | Roche, Abbott, Thermo Fisher, Qiagen, Danaher, and Randox |
| TB reagents | Danaher, Molbio, Qiagen, and Bio-Rad |
| HIV, HBV, and HCV reagents | Abbott, Qiagen, Roche, and Altona |
| Oncology reagents | Qiagen, 3B BlackBio Biotech, Roche, and Siemens (Fast Track) |
| H1N1 reagents | Siemens (Fast Track), Qiagen, and Hi-Media |
| Reagents for other Infectious diseases (including HPV, CT/NG, STI markers, dengue etc ) | Bio-Rad, PerkinElmer, Beckman Coulter, bioMérieux, Tulip Diagnostics, CPC, and Medsource Ozone |
| ADI Media Research | |
This has given a huge impetus to the molecular diagnostics market. While as yet data is not available on how large the market became, estimates indicate that the escalation in demand from extraction machines, extraction kits, amplification test equipment, and RT-PCR kits is ₹7000 crore in 2020, translating into a total Indian molecular diagnostic of ₹7264 crore.
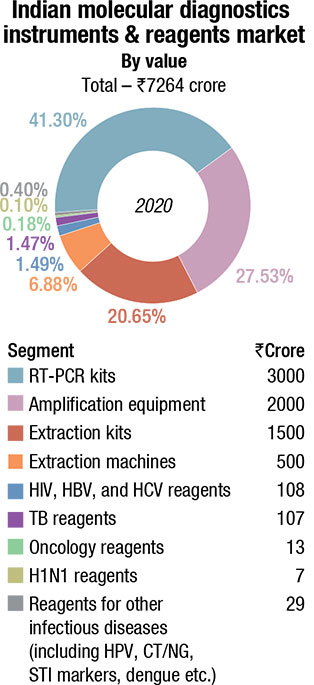
And since now capacities have been created, and huge investments made, apart from the continuity in COVID-19 tests 2021 onwards, molecular diagnostics is expected to start a new chapter, where tests for dengue, malaria, chikungunya, etc., will be conducted on the molecular diagnostic techniques.
Next-generation techniques have begun to make their way into the clinic in a range of diagnostic applications, including prenatal screening for genetic disease, transplant typing, and risk assessment and treatment selection in oncology. While traditional molecular diagnostic techniques, such as PCR (polymerase chain reaction) and ISH (in-situ hybridization) will remain relevant for years to come, many emerging applications require the rich information NGS can offer. New associations between the broad/deep genetic information NGS can provide and disease prognosis or treatment are being reported with increasing frequency. The next huge impetus is expected from these tests, albeit costs of NGS continue to plummet.
 Increasing relevance of molecular diagnostic in COVID-19
Increasing relevance of molecular diagnostic in COVID-19
Thomas John
Managing Director,
Agappe Diagnostics Ltd.
Earlier years, molecular diagnostics was not acceptable to healthcare fraternity due to its higher cost, chances of cross contamination, and lack of properly trained technicians. But COVID-19 has transformed this technology popular these days. Especially, for infective range of disease testing, normal smaller labs cannot afford to accommodate this technology. Because of continuous research and development process, molecular biology tests are now cost-effective, in spite of their high upfront expense, and flexible enough to manage rapidly to new threats. In resource-limited environs, molecular equipment is commonly reserved for special or reference-based testing. But broader application of these resources will produce economies of scale and make local production of testing materials commercially viable in the low-income countries, after the COVID-19 outbreak.
COVID-19 placed the basis for a viable socially essential concept of RT-PCR from research-based usage to mass-scale lab use for confirmatory screening purpose. From March 2020, across the world, the number of molecular-based systems skyrocketed to cover the lion’s share of population screening for SARS-CoV-2 in clinical specimens.
Molecular diagnostics techniques like RT-PCR, RT-LAMP, and sequencing play a vital role in containing the pandemic by diagnosing the symptomatic individuals, screening asymptomatic individuals, monitoring the disease spread, screening predisposition population, and generating genomic data for statistical analysis. The data generated will give an overall picture of the pandemic and help in planning strategies.
For a moderate and small lab or for a hospital, where sample load is low to moderate and need reports generated on emergency basis within very less TAT, the RT-LAMP technology is the best solution. Sequencing technology is highly expensive and is suitable for R&D institutes to identify new variants and monitor the spillage pattern of the pandemic, and may not be applicable for a diagnostic lab. RT-PCR is appropriate only for a lab that handles huge sample load that has enough time for reporting. RT-LAMP platform is very economical compared to RT-PCR. It can take up broader extent of infectious outbreaks like Leptospira, human papillomavirus, and malaria with lowest TAT and on economical scale with limited initial investment.
A few nations have effectively contained COVID-19 better than other nations by detailed statistical analysis of their data, predicting, and designing the strategy to use molecular diagnostic methods according to their populations and effective isolation techniques. Yes, it’s true – Necessity is the mother of invention.
The global MDx market, including new tests for COVID-19, exceeded USD 13 billion in 2020, according to Kalorama Information. That is about USD 5 billion larger than 2019, and reflects the large volume of sales for COVID-19 molecular tests in the US and worldwide.
COVID-19 testing is slated to continue into 2021 if present disease trends continue. While it is possible COVID-19 tests will be panelized, that is added to existing tests in combination with flu and other respiratory conditions, and while it is also true that the market may not support the kind of emergency crisis sales of 2020, the influence of COVID-19 on molecular diagnostics should last a few years. It already has boosted the respiratory and healthcare infection molecular testing markets and has generally increased public awareness of PCR testing.
The news is not all positive from a revenue perspective. Molecular testing on COVID is paired with declines in more traditional molecular tests as patients avoided doctors, and continue to reduce in-person doctor visits during lockdown. Down-but-not-out segments include cancer, histology, and inherited diseases, which are expected to continue to grow, perhaps surge, later in the year. Prior to COVID-19, liquid biopsy was one of the stars of this segment. Favorable regulatory policies and promising studies will enhance companion testing. New LDT tests in inherited disease testing in particular, using IVD supplies, which includes non-invasive prenatal testing (NIPT), is driven by the demand in China. Even though the demand is for laboratory test services and not for IVD products, the demand for instruments, kits, and consumables that are approved by regulatory agencies is driving the segment. However, the market opportunities may be challenging to access by foreign IVD companies due to the regulations promoting domestic companies.
The molecular diagnostics field, up until recently, has been dominated by large firms, such as market leader, Roche, followed by Cepheid/Danaher, bioMérieux, Qiagen, Hologic, BD, Siemens, and Luminex. While many of these companies are focused on competitive strategies (e.g., sophisticated automation for molecular testing, test menu expansion) to maintain their position, emerging competitors are also entering the market by developing next-generation technologies. The global COVID-19 pandemic has accelerated these and other new approaches for molecular diagnostics to enter the market, through substantially reduced regulatory hurdles, with many novel technologies coming out of research laboratories.
Many of the vendors making COVID-19 tests also make tests for other infectious diseases, such as HIV and zika. Those focused on the novel coronavirus may disappear when the crisis is over, but others may remain. There is no shortage of new competitors entering this market, and even if a solution emerges for COVID-19, it is logical to expect that many of these new vendors will continue to develop lab test products for other conditions and some will be on standby with production knowledge and facilities for the next pandemic.
While necessity is the mother of invention, anomalies have formed the basis for most disruptive discoveries that seed innovations in the sciences. They provide the impetus for paradigm change within a field and reflect differences between observed and theoretically expected data. The coronavirus pandemic was such an anomaly that spawned innovation in the molecular diagnostic testing market and initiated a paradigm change in public health policies, regulatory hurdles, and consumer views of point-of-care (POC) testing. Prior close calls with other viruses, including SARS, MERS, and Ebola, should have prepared the world for the coronavirus pandemic. Yet, many nations, including the United States, found themselves largely unprepared.
Countries responded to the unprecedented challenge of the coronavirus pandemic differently, but in each case, innovation was at the core of the response. After the authorization of the CDC test via emergency use authorization (EUA) from the US FDA on February 4, 2020, lab-developed tests from Clinical Laboratory Improvement Amendments (CLIA)-certified labs were authorized soon thereafter. Multiple commercial diagnostic companies rapidly validated and received EUA for SARS-CoV-2 diagnostic assays on existing platforms, which enabled private testing to rapidly outstrip public health department testing.
Nucleic acid detection technology. Nucleic acid detection is an important diagnostic tool for clinical diagnosis, segregation, rehabilitation, and discharge of patients, and was also the gold standard for the detection of 2019-nCoV infection in the early stage of the epidemic. Current nucleic acid detection methods include RT-PCR, isothermal amplification, and high-throughput sequencing. At present, specimens tested by commercial nucleic acid kits mainly comprise throat swabs, oropharyngeal swabs, nasopharyngeal swabs, sputum, and alveolar lavage fluid.
RT-PCR technology. At present, RT-PCR nucleic acid detection serves an irreplaceable role in the diagnosis of 2019-nCoV and is the most important molecular diagnostic method in the early stage of the epidemic. However, there are limitations due to tedious, time-consuming operation, required biosafety laboratories ranked Class II or above, centralized inspection and shortage of personnel, and qualified biosafety sites in the epidemic area. Furthermore, there are shortcomings in responding to the rapidly increasing demand for the diagnosis of patients with suspected 2019-nCoV pneumonia and asymptomatic infections.
High-throughput sequencing technology. Gene sequencing is the most accurate and reliable technology for the detection of viruses and other pathogenic emergency infectious diseases. Additionally, it is the only method to dynamically track genome variation in pathogens.
 Advancements in molecular testing
Advancements in molecular testing
Dr Manoj Chugh
Vice President – R&D,
Transasia Bio-Medicals Ltd.
Molecular diagnostics (MDx) is witnessing a fast-paced growth for detection of infectious micro-organisms as well as ailments like oncology. Laboratories are relying on MDx for improving efficiency, early diagnosis, and accurate detection.
Polymerase Chain Reaction (PCR)
PCR applies primer-mediated enzymatic amplification of DNA to synthesize new strands of DNA, complementary to the targeted template strand. Quantitative real-time PCR (qPCR)/RT-PCR is gaining popularity. This technology requires less than 5 hours, is simple, reproducible, and offers a quantitative output.
Isothermal amplification methods
Current advancement in qPCRs/RT-PCRs has led to the adoption of isothermal amplification methods, including loop-mediated isothermal amplification (LAMP), and polymerase spiral reaction (PSR). LAMP is a unique nucleic acid amplification technique that amplifies few copies of DNA into billion copies within an hour under isothermal conditions with greater specificity.
Next-generation sequencing (NGS) technology
NGS offers broad investigations of the bacterial genomes. It can generate high-quality and lower-noise background sequence data and is a preferred tool for identification of single-nucleotide polymorphisms (SNPs) for detecting mutant strains/ variants.
POCT – Molecular
Diagnostic advancements are further enabling development of systems with cartridges that can be run without stringent clean room requirements, while minimizing contamination risks, associated with molecular assays. Molecular assays, based on CRISPR and CAS9 and lateral-flow detection, could lead the way and gain market share in the future.
The advantage
Molecular assays can target multiple gene segments in one reaction. Multiplexing provides several answers to clinicians for identifying the pathogen, and quantitative data helps in therapeutic drug monitoring. Industries find it easy to scale molecular assays, as oligosaccharides, enzymes, and probes are readily available and once optimized, menu expansion is easy.
Stringent transportation and storage requirements make these tests less accessible and affordable for laboratories in rural areas. However, more and more companies are building capabilities to lyophilize reagents to mitigate these challenges.
To conclude, Indian diagnostic companies are geared up to match MNCs for developing advanced molecular products.
 Molecular diagnostic in rapid disease diagnosis
Molecular diagnostic in rapid disease diagnosis
Rajesh Patel
CEO – IVD, India,
Trivitron Healthcare
Molecular biology methods have revolutionized the diagnostics industry and have now become an important part of conventional diagnostic testing and specimen processing. Molecular diagnostics (MDx) procedures are now frequently used as a guide for patient management, from disease diagnosis to treatment, particularly in the field of infectious diseases, birth defects, and oncology.
The diagnosis of viral infections takes longer turnaround time (TAT) due to the traditional cell culture system that requires huge cost, time, and even skilled person to conduct the test. Molecular biology PCR (polymerase chain reaction) technology has now improved the detection of a number of these viruses. The nucleic acid amplification and detection has now switched diagnostic industry to molecular diagnostic to rely on. The increased demand for genetic and genomic information for diagnosis, research, and vaccine development has led to the rapid expansion of molecular techniques within diagnostic and clinical laboratories.
MDx methods are having great public health importance as they have progressed beyond the identification of the target organism, as they help determine their resistance genes and even provide strain characterization following genotyping. With the help of molecular biology, the diagnosis and treatment for certain microbial diseases have been improved as this technique enables viral resistance detection and viral load testing that help to effectively monitor their response toward the antiviral therapies.
Several infectious diarrhea and respiratory diseases are caused by viruses worldwide as compared to bacteria and other disease-causing pathogens. PCR detection is one helpful test due to the ability to rapidly screen for many respiratory viruses. Significant respiratory viruses such as SARS-CoV detection were also possible with PCR test. PCR testing of respiratory specimens and other respiratory viruses crucially helped to detect the number of suspected cases affected with COVID-19. The diagnosis of coronavirus and even viral diarrheal disease has now been improved with PCR detection.
The pandemic has made us realized the infectious disease emergencies, and the importance of rapid and accurate identification of the causative agent, to optimize anti-microbial therapy promptly that helps to treat the concern at the initial phase.
Trivitron Healthcare R&D and manufacturing facilities have successfully developed many molecular biology diagnostic kits, reagents, and equipment with a wide range of applications that includes diagnosis and monitoring of infectious diseases, genetic disorders, oncology, and various other disorders.
LAMP technology. Developed in 2000, LAMP is a fast and highly specific technology for gene amplification under constant temperature conditions. RT loop-mediated isothermal amplification (RT-LAMP) combines RT with LAMP, can be used directly for RNA detection, and has previously been used in the identification of various respiratory RNA viruses, including SARS-CoV and MERS-CoV. Based on this, by adding a fluorescence quenching probe (QProbe), fluorescence RT-LAMP technology can be used for the detection of MERS-CoV. In order to make the detection of LAMP amplification products more accurate, the combination of nucleic acid detection and immunogold labeling technology has resulted in an improved RT-LAMP-combined nucleic acid strip detection technology (RT-LAMP-NAD), which has been used for the detection of Ebola virus.
Although LAMP technology has the advantages of simplicity, sensitivity, specificity, speed and is inexpensive and has low hardware requirements, the development of a kit using this technology is more complicated than an RT-PCR kit and involves multiple pairs of primers. Therefore, the development and clinical application of LAMP in 2019-nCoV pneumonia epidemic is slower than RT-PCR.
Recombinase-aided amplification technology. RAA technology utilizes recombinases, single-stranded binding proteins, and DNA polymerases to perform nucleic acid amplification under isothermal (37˚C) conditions. RAA technology is relatively new in the current nucleic acid detection technology field. The advantage of rapidity, sensitivity, and specificity of RAA technology may aid in the detection, screening, and isolation for suspected 2019-nCoV infections.
Nucleic acid mass spectrometry. Nucleic acid mass spectrometry is a novel type of soft ionized biological mass spectrometry technology that has been developed recently based on matrix assisted laser desorption ionization-time of flight technology and is very simple and efficient. This procedure integrates the high throughput of chip technology, and the high sensitivity of mass spectrometry technology, without the requirement for complex biological information analysis and is mainly used for the detection of known mutations. A single reaction of nucleic acid mass spectrometry can perform 20–50 PCR amplifications simultaneously and can detect dozens of pathogens at once. Nucleic acid mass spectrometry is a very useful tool for differential diagnosis of respiratory infections.
Nucleic acid mass spectrometry has high-throughput analysis, is simple to operate, and is inexpensive; nucleic acids are difficult to ionize, are unstable, and easily generate fragments. This makes it difficult to parse spectrum data. It is necessary to continuously improve the resolution of the detector to promote its use.
Protein detection technology. It is mainly divided into pathogen antigen detection and host antibody detection. Commonly used methodologies include colloidal gold, immunofluorescence chromatography, chemiluminescence, and ELISA.
Point-of-care testing. The current technology platform used by the majority of point-of-care testing (POCT) integrates nucleic acid extraction, amplification, and detection on a microfluidic chip that reduces detection complexity. POCT has the advantages of rapid results, unrestricted test sites, and low professional skill requirements for operators. Therefore, the research and development of POCT nucleic acid detection technology is likely to be the general direction of future development of testing. Operators only need to add samples, such as swabs or blood, into the slot on sample in, result out requirement, which will significantly simplify the detection process. POCT automatically completes nucleic acid amplification, signal collection, and result analysis in a short time. However, POCT requires improvement due to lack of authoritative control experiments and lack of uniform national standards for manufactured products.
Molecular diagnostic techniques and platforms are playing a larger and more critical role in all areas of anatomic and clinical pathology. In the last decade or so, the clinical laboratory has seen an explosion in the available menu of tests based upon DNA and RNA analysis.
In the future, molecular diagnostic research of 2019-nCoV infections will speed up sample preparation, increase detection throughput and accuracy, improve detection automation level, and develop novel technologies with low requirements and low costs for equipment and testing personnel. Due to antibody preparation requiring additional time, faster breakthroughs are expected in pathogen nucleic acid detection technology.
CRISPR – The future of molecular diagnostics?
CRISPR-based systems are well known for their use in gene editing and gene therapy applications due to their ability to cleave or cut nucleic acids. More recently, researchers have begun using the bacteria-derived technology to develop the next generation of rapid, accurate, and inexpensive molecular diagnostic technologies that can detect everything from antibacterial resistance and viral outbreaks to cancer-causing mutations in circulating tumor cells.
In terms of molecular diagnostics, CRISPR-based systems are ideal to detect the nucleic acid sequences of different pathogenic strains with single base specificity. For example, Cas9 and Cas12 proteins target DNA, while Cas13 targets RNA. This is a major advantage over the generated primers used to amplify target sequences in PCR, which are prone to off-target effects and non-specific amplification.
To create diagnostic applications, researchers have linked target sequence binding with readout, such as color changes, in the case of paper-based lateral flow assays, or fluorescence. In terms of fluorescence, researchers took advantage of the fact that once the Cas12 and Cas13 enzymes cleave their target sequence; they continue to cleave neighboring nucleic acids indiscriminately. Thus, by including nucleic acid reporters that fluoresce when cleaved in the sample, researchers can link cleavage of the target sequence to a fluorescent signal.
 Prof (Dr) Balram Bhargava
Prof (Dr) Balram Bhargava
Director General,
ICMR
“We have seen that exponential increase in testing led to early identification, prompt isolation and effective treatment of COVID-19 cases. This testing milestone is testimony to the fact that India has been successful in implementing strategy of 5T approach Test, Track, Trace, Treat, and use of Technology efficiently, which will enable us to contain the spread of the pandemic. Further, enhanced production of diagnostic kits has made India Atma Nirbhar, which has resulted in reduction of costs and improved availability of testing kits.”
In addition to improving the accuracy of nucleic acid tests, CRISPR-based molecular diagnostics are ideal for POC testing in low-resource settings as they do not require a complex laboratory setup; the reactions use simple reagents and do not require thermocycling, unlike PCR-based technologies.There are currently a variety of CRISPR-Cas diagnostic platforms being developed to diagnose infectious and noninfectious diseases.Viral. An advantage of Cas13-based diagnostics is that they directly target RNA. An RT-PCR test for an RNA virus requires the RNA to be amplified into DNA before PCR, which can lead to non-specific amplification and false-negative diagnosis. Introduced in 2017, the Sherlock (specific high-sensitivity enzymatic reporter unlocking) platform uses Cas13a and a fluorescent reporter to detect target RNA molecules. More recently, researchers enhanced Sherlock to detect viral genetic material directly from patient serum, urine, and saliva samples in less than 2 hours without the use of any other instruments. This protocol has been used to diagnose flaviviruses, such as zika, dengue, West Nile, and yellow fever.Similarly, the Detectr (DNA endonuclease-targeted CRISPR trans reporter) method, which uses Cas12a to target DNA, is also linked to a fluorescent reporter, and has been used to detect human papillomavirus in patient samples.
 Prof (Dr) Viyatprajna Acharya
Prof (Dr) Viyatprajna Acharya
Dept. of Biochemistry,
Kalinga Institute of Medical Sciences, KIIT (DU) Bhubaneswar
“Track-test-treat being the call, the major thrust of innovation came unto the diagnostics sector during this COVID pandemic. Since RT-PCR requires expertise and sophisticated instruments, and has a longer reporting time, many other techniques like RT-LAMP, lateral flow assay (LFA), CRISPR COVID, have also appeared as alternative techniques.
RT-LAMP (Reverse transcription loop-mediated isothermal amplification) can be best used for rapid screening and diagnosis as it can let out the result within 40 minutes. The reaction is carried in isothermal condition, has good yield, and needs a simple detector. Some experiments have found this technique 10 times more sensitive than RT-PCR. However, the risk of cross-contamination due to aerosol formation during running might be its limitation. However, so far it has proven itself as a rapid, sensitive, and specific test for diagnosing SARS-CoV-2.”
Both of these platforms are versatile and can be adapted to a variety of pathogens in response to novel outbreaks. Researchers recently used Detectr and Sherlock to develop a test for SARS-CoV-2, the latter of which could produce results in an hour.
Bacterial. The Flash-NGS platform, which combines Cas9 and NGS technologies, can target thousands of bacterial sequences linked to antibacterial drug resistance. In clinical settings, Flash-NGS has been used to detect MRSA (Methicillin-resistant Staphylococcus aureus and VRE (vancomycin-resistant E. faecium) infections.
Likewise, researchers have used Cas12a to develop a highly sensitive test for mycobacterium tuberculosis (Mtb). The test, called CRISPR-MTB, had better sensitivity (79%) than Mtb culture (33%), while maintaining a specificity of 98 percent. In addition to better sensitivity, which reduces the chance of false-negatives, because CRISPR-MTB does not require culture, patients could receive reliable results sooner.
Cancer and genetics. Identifying genetic mutations, such as single nucleotide polymorphisms (SNP), currently requires benchtop instruments or genome sequencing, but the specificity of CRISPR-based diagnostics has the potential to change that. For example, during the zika virus epidemic, researchers quickly developed a Sherlock-based test to detect an SNP associated with fetal microcephaly in patients with zika virus. Moreover, producing results in only 15 minutes, the CRISPR-chip platform has been used to detect the deletion of two exons associated with Duchenne muscular dystrophy.
The highly specific and sensitive nature of CRISPR-based tests would be especially useful for the detection of cell-free DNA and circulating tumor cells, which are present in very small amounts in serum, requiring a highly sensitive test.
Toward CLIA certification. Though PCR-based diagnostics are currently the gold standard, since their introduction in 2017, CRISPR-based diagnostics have quickly evolved with several advantages, namely speed, simplicity, and lower costs. They are ideal for POC settings, where rapid results can accelerate access to treatments and help prevent the spread of infections.
Though CRISPR-based diagnostics offer accessible readouts, and do not require complex laboratory facilities with benchtop thermocyclers, many of the other limitations of PCR-based diagnostics, such as reliable access to PPE, sample reagents, and nucleic acid extraction kits still also apply to CRISPR-based tests. These challenges must be overcome to produce a one-step diagnostic test that meets the Clinical Laboratory Improvement Amendments requirements. Even so, there is no doubt that portable CRISPR-based tests will soon revolutionize the field of clinical diagnostics.
For the first time in the history of the diagnostic laboratory, molecular pathology and diagnostics are extending the range of information available to physicians, pharmacists, geneticists, forensic scientists, research scientists, and other healthcare professionals.
In the future, novel epidemics or pandemics may be inevitable. It is crucial that pandemic-prevention agencies perform further research on pathogen differential diagnosis technology to improve testing times, provide definitive diagnoses, and diagnose diseases differentially with similar clinical manifestations. There are various types of pneumonia-related pathogens, including 2019-nCoV, SARS-CoV, influenza virus, parainfluenza virus, adenovirus, respiratory syncytial virus, rhinovirus, mycoplasma, and chlamydia. Considering RT-PCR results are time-consuming and laborious, there is an urgent need for medium-throughput detection technology for the differential diagnosis of 2019-nCoV and non-2019-nCoV conditions. In the future, it is necessary to focus on the development of high-throughput and low-cost differential diagnostic technologies. Furthermore, the development of detection technologies and supporting reagents that can simultaneously rapidly detect dozens of pathogens will be beneficial.












