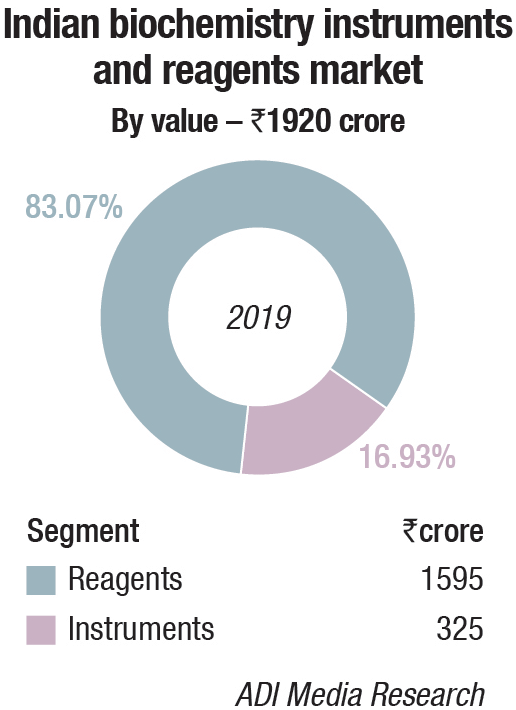
Biochemistry Instruments and Reagents
Embracing automation in biochemistry

Gone are the days of radio immune assays, using a gamma counter; the industry now has the CLIA technology with better sensitivity, specificity, and reproducibility.
Over the last few decades there has been a significant decrease in the number of analytical errors in clinical laboratories much to the fact that laboratories are required to meet very high standards. The technological developments and scientific innovations in the field of clinical chemistry from the early 1950s to date have been vast, enhancing laboratory capabilities, and providing the necessary support to clinicians and laboratories to improve patient diagnosis and treatment.
Right from batch analyzers in routine chemistry and immunoassay analyzers to the integrated fully automated systems with a very high throughput, the biochemistry labs have become efficient with very low errors in the analytical phase of laboratory testing.
Today presents a new world of automation—a complex integration of robotics, liquid handling, and numerous other technologies with the fundamental purpose of saving time and improving performance through the elimination of human error and reduced risk of cross-contamination.
Now technologically advanced analyzers are capable of automating the process of repetitive sample analysis that were earlier done by lab technicians manually. Industry players are developing biochemistry analyzers with the feature of positive identification that decreases the process of repeated pathogen testing. This feature is important for samples with low volume, such as neonatal units.
Fully automated biochemistry analyzers can perform functions, such as recognition of sample and reagent bottles, cap piercing, tube sampling, dilution, and automatic re-run, which reduces human effort and time, and require lesser volumes of reagent and sample. These analyzers aid scientists in measuring the concentration of any substance in a reaction mixture. The implementation of IT and automation in these analyzers is expected to drive the global biochemistry analyzers market growth.
Furthermore, integrated systems that combine immunochemistry and biochemistry tests are gaining huge attention. It increases the workflow efficiency and delivers fast turnaround and high throughput. It also helps in achieving increased instrument capacity by connecting different analyzer units with a single sample-presentation mechanism. These systems are offered by Roche, Siemens, Abbott, and Beckman Coulter.
On the other hand, besides pathogen testing, biochemistry analyzers are used for drug monitoring, drug abuse detection, and many more applications. Due to such technological advancements in the field of professional diagnostics, the applications of biochemistry analyzers that were initially restricted to the detection of infectious diseases are now venturing into other areas as well. As a result of this technological evolution, the diagnostics tests are witnessing a boost in their demand.
 “Healthcare dependents on diagnostic reports include biochemistry. Perception that expertise in instrumentation and reagents has peaked needs a rethink. Irrespective of instrument, reagent or lab used, variation should be minimized. Focus should be on measurement uncertainties, traceability of calibrators, standards, quality control, and reagents for quality reporting.”
“Healthcare dependents on diagnostic reports include biochemistry. Perception that expertise in instrumentation and reagents has peaked needs a rethink. Irrespective of instrument, reagent or lab used, variation should be minimized. Focus should be on measurement uncertainties, traceability of calibrators, standards, quality control, and reagents for quality reporting.”Dr Babli Dhaliwal
CTO, Trident Diagnostics
Selecting a chemical analyzer
Selecting a chemical analyzer depends on the types of tests the laboratory needs to run and the throughput required. Sample handling, degree of automation, footprint, operational costs, turnaround time, STAT capabilities, service dependency, and whether the analyzer can handle micro volume samples are also important considerations.
With both semi-automated and fully-automated analyzers, laboratory informatics and process management software has increased heavily over the last two decades. Significant implementation of quality control and interfacing automatically and reliably transmit data to and from various systems. This improves both quality and productivity, creating straightforward operations, requiring users to have minimal training requirements for use.
Today, the advancements in quality control software are designed to allow laboratories to meet industry and international standard requirements while ultimately ensuring accurate and reliable instrument performance.
Way forward
The history of automation in the clinical laboratory is long and varied. Manual testing is clearly of the past century for a modern laboratory except for a few very specialized tests.
Even if a laboratory’s workload is of such a low volume and TAT is not a concern, the inherent variability of manual procedures makes them nonviable in comparison to modern automated methods, and it is a given that laboratories will inevitably adopt automation.
The dilemma for clinical chemists is to decide what kind of automation and what extent of automation is suitable for a given facility. The advantages of automation are undeniable. The challenge is for laboratories to embrace the right kind of automation to best meet their specific patient testing needs. This starts with a careful analysis of a laboratory’s current process and optimizing it before making any automation decisions. It can be tempting to make the jump to some level of automation without first performing a process analysis, but it is a risky move. It is better to fully understand what kind of automation is needed, and adapt the right kind of automation to meet the need.
There are myriad options available, from stand-alone integrated systems, to pre- and post-analytical modules that can be mixed and matched with analytical units, to total laboratory automation. This is definitely a situation in which one size does not fit all. Automated systems must match the specific work volumes and needs of each laboratory.
Blindly copying the automation solution that is acceptable for another laboratory is discouraged. At the same time, standardization of automation throughout a network of laboratories is highly desirable. TLA is the ultimate automation development, but not necessarily the right solution for a laboratory. A facility may find specific, targeted automation to be a better option.
Not to be overlooked is the availability of middleware, the sophisticated software that links analyzers and the LIS and offers the ability to take test results, combine them with patient demographic data and principles of evidence-based laboratory medicine, and offer value-added clinically useful information to healthcare providers. Middleware is applicable to every level of automation.












