Biochemistry Instruments and Reagents
From manual beginnings to an automated revolution

While robots may not be coming for the biochemist’s job just yet, it may be a different matter once automation is coupled with advanced AI. One thing is for certain, automation continues to be the future of biochemistry testing.
The conclusion of every calendar year is a good moment to take stock of where the industry has been and, more importantly, where the market is going.
It is no exaggeration to say that the year 2021 was both a time of tremendous challenges for clinical laboratories – and the broader healthcare industry – as well as a time of significant opportunities. And, while the global Covid-19 pandemic will undoubtedly eclipse 2021 and 2020, what does 2022 hold for the industry? Is it going to be the same again? Or something significantly different?
There is little doubt that Covid will continue to touch a large portion of the patient population, particularly in the aftermath of an infection.
Chronic or Long Covid will become an increasingly important issue in healthcare around the globe. Legislation is likely to be enacted to provide funding for research, testing, and treatment for long Covid patients. Significantly, this will mean that the approach to testing will have to change.
While diagnostic testing to detect the presence of Covid will continue – albeit at a significantly lower rate – novel testing will be needed to address the rapidly increasing number of chronic Covid patients, whose immune systems have been compromised, resulting in a wide range of debilitating symptoms. This need represents an opportunity for IVD companies and laboratories to develop and offer specialized testing that will better identify, evaluate, stratify, and characterize long hauler patients for actionable treatment options.
Covid-19 pandemic is expected to continue to positively impact the market in 2021 and 2022, which is forecast to grow at a higher pace comparing to pre-pandemic levels (CAGR 11%–13%) due to a progressive reduction of swab tests, but an increase in serological ones to map antibodies presence after vaccination.
As regards technology segmentation, Covid-19 has positively impacted biochemistry, immunoassay, and molecular diagnostics/genetics, while there has been a negative impact on other segments due to decline in the number of installed bases and routine testing.
Over the past 80 years, clinical chemistry has played a central role in the determination of the structures and chemical reactivity of the building blocks used by all organisms, providing the molecular framework to elucidate the pathways involved in central metabolism. The pandemic played a pivotal role in the growth of biochemistry, which increased the demand for special parameters like LDH, ferritin, D-Dimer, CRP, and reduction in manual or human interventions. Diagnostic companies are working on test kits that have great cross-platform correlation. Clinical chemistry platforms, on the other hand, will continue to be the favored choice because to their technical superiority.
The pandemic has also led to greater emphasis on diagnosis, and laboratories are looking at ways to increase efficiency and provide cost-effective operations. Manufacturers are now developing biochemistry analyzers with multiplexing. Such analyzers possess the feature of positive identification that reduces the process of repeated pathogen testing. This becomes a critical feature in cases of samples that have low volume, such as neonatal units. This type of system with shorter turnaround time gives advantages of high clarity and result accuracy. The feature of positive identification helps acquire accurate results in shorter run time by avoiding the inclusion of too many targets.
In addition, manufacturers are offering a wide range of features in an automated clinical chemistry analyzer, with a multitude of benefits for error-free reporting while increasing test volumes and can ultimately provide a positive impact on patient safety. The continuous auto-loading mechanism minimizes operational intervention. Similarly, there is a growing trend for triple chemistry reagent systems for performing specialized tests, which eliminate the need for external mixing of reagents.
Trends in clinical chemistry
 Thakur Abhishek Singh
Thakur Abhishek Singh
Group Product Manager – Biochemistry,
Transasia Bio-Medicals Ltd.
With a wide range of parameters being screened for preventive and therapeutic monitoring, clinical chemistry remains one of the most important segments in diagnostics. In India, clinical chemistry registered a share of 20–25 percent in 2020, and is the largest segment estimated at approximately ₹3000 crore, with reagents having almost 80 percent of market share. The pandemic has led to greater emphasis on diagnosis, and laboratories are looking at ways to increase efficiency and provide cost-effective operations.
Increased testing for critical parameters. There has been a considerable rise in testing for CRP, ferritin, LDH, etc., post Covid. Diagnostic companies are developing test kits, exhibiting excellent correlation on multiple platforms. Having said that, clinical chemistry platforms continue to remain the preferred choice due to their technical superiority.
Besides these, therapeutic drug monitoring, a branch of clinical chemistry that specializes in the measurement of medicine in blood, is another growing field.
Technological advancements. Automation is being routinely implemented by labs for greater efficiencies. Diagnostic manufacturers are offering a wide range of features in an automated clinical chemistry analyzer, with multitude of benefits for error-free reporting while increasing test volumes, and can ultimately provide a positive impact on patient safety. Continuous auto-loading mechanism minimizes operational intervention. Similarly, there is a growing trend for triple chemistry reagent systems for performing specialized tests, which eliminate the need for external mixing of reagents.
Integration of LIS and IoT. Along with analytical automation, there is a growing rise in the integration of laboratory information system (LIS) to evaluate the pre- and post-analytical phases, including reading specimen ID, monitoring the analyzers with respect to predefined algorithms, post-analytical processes, such as re-runs, dilutions, and quality control. Besides, diagnostic manufacturers are upgrading their clinical chemistry instruments through integration of IoT and AI to provide predictive maintenance and remote monitoring.
Integrated modular systems. Multidisciplinary convergence is leading the way to integrated diagnostics, especially in laboratories with high workload. Consolidation and integration aids in gathering and compiling information from different analyzers onto a single platform. This has an immediate positive impact on the efficiency, allows access to multiple disciplines at one go, and enhances the overall performance and TAT.
On the other hand, besides pathogen testing, biochemistry analyzers are used for drug monitoring, drug abuse detection, and many more applications. Due to such technological advancements in the field of professional diagnostics, the applications of biochemistry analyzers that were initially restricted to the detection of infectious diseases are now venturing into other areas as well. As a result of this technological evolution, the diagnostic tests are now witnessing a boost in their demand.
Initially, biochemistry analyzers were used for repetitive analysis that consumed a lot of reagents. This has now changed and due to the replacement by discrete working systems, low-volume reagents are now being used. The new instruments are able to automate repetitive sample analysis steps that would have otherwise been done manually by a lab technician.
Automation in biochemistry – Way forward
 Shobhit Jain
Shobhit Jain
Biochemistry, Urinalysis, Marketing Events and Branding,
Sysmex
Indian in vitro diagnostic (IVD) industry is growing at a phenomenal pace with excellent potential to emerge as a global manufacturing hub in medical devices. Especially, after the success of the government’s Make in India initiative, many multinational companies have started planning to initiate manufacturing in India. After China, Japan, and South Korea, India is the fourth largest market in IVD in Asia.
Biochemistry segment continues to dominate IVD market with largest contribution in the growth of Indian IVD market. Indian biochemistry market in 2020 is estimated at ₹1990 crore, in which reagent accounts for 83 percent and instrument accounts for 17 percent. High prevalence of chronic diseases, increasing use of point-of-care (POC) diagnostics, rising awareness, and acceptance of personalized medicine and companion diagnostics, are the major growth factors.
The current pandemic also played a pivotal role in the growth of biochemistry, which increased the demand for special parameters like LDH/ferritin/D-Dimer/CRP, and reduction in manual or human interventions.
The journey of biochemistry started with very basic measurement systems like colorimeter and flame photometer which were taken over by semi-automatic analyzers and batch analyzers in the next decade.
Furthermore, with technological advancement (automatic on-board hemolysis for HbA1c, permanent cuvettes, onboard laundry and cooling, clot detection, bidirectional interfacing, Westgard QC reporting systems), integration (modular platform), tools for preanalytical, analytical, and post-analytical, the future of biochemistry lies in automation and total lab automation (TLA).
Other socio-economic factors like growing population, increase in health awareness in Tier-II and Tier-III cities, increase in medical tourism, resulting in increase in sample load, etc., have resulted in a growing need for automation, which enables fast, cost-efficient, and high-quality testing.
In addition, labs are also expanding their business by expanding menu, opening new branches, and joining hands with existing market leaders, creating good demand for automation. To conclude, from small-size to high-end laboratories, private to public sector hospitals, there is a high demand for automation, and it will be exponentially growing in future.
Moreover, as a result of the convergence of system engineering, automation, and IT technology, a significant change has been brought in the biochemistry analyzers market. The use of ELISAs for clinical testing within a laboratory is time consuming and demands more personnel and resources. However, moving from ELISA technique to an automated biochemistry method increases time and personnel efficiency considerably, and this leads to cost effectiveness as well.
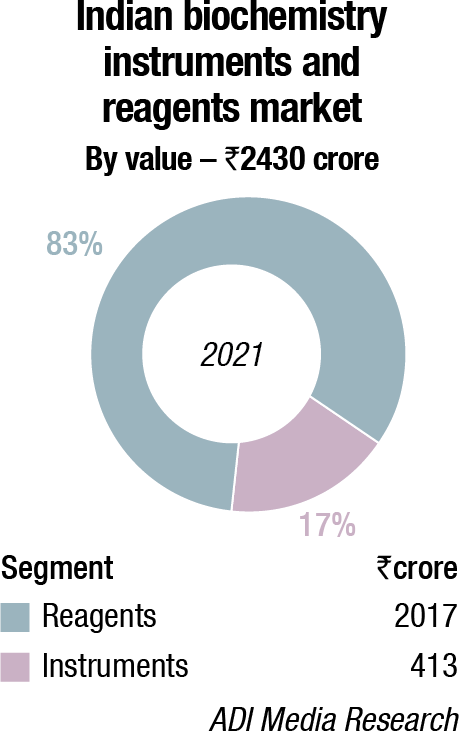
In 2021, the Indian biochemistry instruments and reagents market is estimated at ₹2430 crore, with reagents continuing to dominate at ₹2017 crore at 83-percent market share. This is a 22.08 percent increase by value and a 24.96 percent increase by quantity, over 2020.
The floor-standing analyzers are estimated at ₹129.34 crore and 925 units; benchtop analyzers at ₹121.84 crore and 2435 units; and semi-automated analyzers at ₹161.82 crore and 23120 units. Almost 80–90 percent floor instruments are on rentals; this figure is much smaller for bench-top. Semi-automated instruments are all procured, with almost none on rentals. The size of the market has been calculated on assigning a monetary value to all the instruments installed, whether placed or sold.
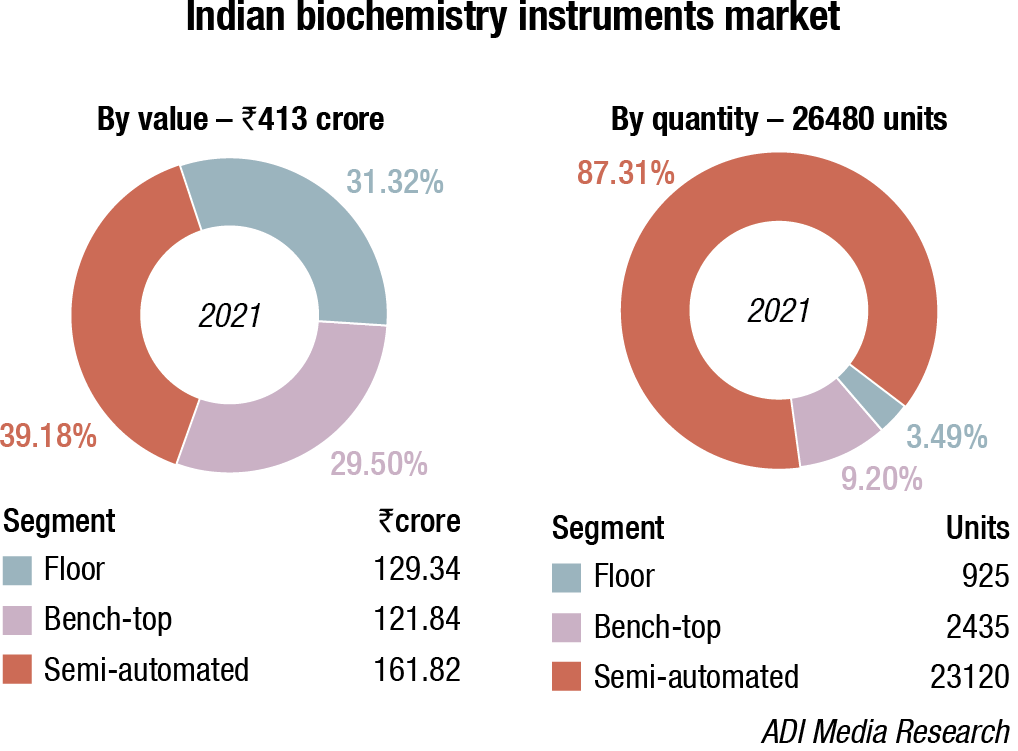
Transasia continues to be a clear leader in this segment. Roche, Beckman Coulter, Siemens, OCD, and Abbott are the other leading brands for reagents. It is estimated that ₹250 crore worth of reagents were consumed as a direct impact of Covid. The current pandemic increased the demand for special parameters like LDH/ferritin/D-Dimer/CRP. By 2022, this is expected to lose its momentum.
|
Indian biochemistry instruments and reagents market |
|||
|
Major vendors* – 2021 |
|||
| FA | SA | Reagents | |
| Tier I | Transasia | Transasia | Transasia, Roche, Beckman Coulter, Siemens, OCD, and Abbott |
| Tier II | OCD and Roche | Mindray and Robonik | Agappe, Randox, Accurex, DiaSys, Biosystems |
| Tier III | Mindray, Biosystems, Beckman, Abbott, Thermo Fisher, Siemens, Meril Diag, and CPC | Agappe, Eurit, CPC, Biosystems, Thermo Fisher, Accurex, Tulip, Microlab, Beacon, and regional brands | Fuji, Mindray, Rapid, and local brands |
| Others | Trivitron, Sysmex, Tulip, Biosystems, and Accurex (Dirui) | ||
| *Vendors are placed in different tiers on the basis of their sales contribution to the overall revenues of the Indian biochemistry instruments and reagents market. | |||
|
|
ADI Media Research |
||
In the fully automated instruments segment, Mindray, OCD, and Roche have aggressive presence. Since Agappe represents Mindray, Canon (Toshiba), TokyoBoeki, Furono, and Dirui for fully automated instruments, the company has not been included in the tier table.
The global biochemistry analyzers market is projected to reach USD 15.2 billion by 2026 from USD 12.3 billion in 2021, at a 4.3 percent CAGR. The rising geriatric population and the increasing prevalence of chronic and lifestyle diseases, growing adoption of point-of-care (POC) testing devices, and rising demand for laboratory automation drive growth in the clinical chemistry analyzers market. Emerging economies, such as China and Japan, are providing lucrative opportunities for the players operating in the clinical chemistry analyzers market.
The reagents segment accounted for the largest share of the clinical chemistry analyzers market in 2021. This is due to the presence of a vast collection of reagents in the market, serving various clinics’ requirements. Most importantly, the reagents are cost-beneficial, have optimal sensitivity, linearity, and accuracy, which as a result, ensure limited performance variations.
As per the testing-type insights, the basic metabolic panels’ segment held a commanding share of the clinical chemistry analyzers market in 2021. This is ascribed to the increasing occurrence of lifestyle-associated diseases, including diabetes, overweight, and heart stroke. Rising consciousness among the public regarding the significance of POC testing and the benefits of basic metabolic panel testing has led to the increasing acceptance of this test.
In 2021, based on end-users, hospitals and clinics accounted for the largest share of the clinical chemistry analyzers market. The increasing number of hospitals worldwide owing to the increasing incidences of diseases and disorders, increasing adoption of analyzers, and rising technological advancements are the major factors driving this segment’s growth.
North America accounts for the largest share of the clinical chemistry analyzers market. The large share of this region can be attributed to the increasing geriatric population, increasing prevalence of chronic and lifestyle conditions, implementation of favorable government initiatives, increasing healthcare expenditure, improved healthcare infrastructure, and the availability of technologically advanced instruments.
The major players in this market are F. Hoffmann-La Roche Ltd., Danaher Corporation, Abbott Laboratories, Thermo Fisher Scientific Inc., Siemens AG, HORIBA Ltd., Sysmex Corporation, Hitachi, EKF Diagnostics, Ortho Clinical Diagnostics, ELITech Group, Mindray Medical International Ltd., Biobase Group, SFRI Medical Diagnostics, Randox Laboratories Ltd., Medica Corporation, Meril Life Sciences Pvt. Ltd., Erba Mannheim, Genrui Biotech Inc., DIRUI Industrial Co. Ltd., Teco Diagnostics, Balio Diagnostics, Snibe Co. Ltd., and AMS Alliance.
Since the beginning of the pandemic, automated biochemistry has moved up the priority list for many companies. Laboratories today are looking for knowledgeable partners to help them apply proven continuous improvement strategies – borrowed from the manufacturing industry – to healthcare. A partner who is able to offer a total laboratory solution beyond instrumentation placement can help the laboratory to achieve its patient care and operational efficiency goals. This includes supporting the use of the instruments, identifying opportunities for automation, detecting workflow gaps, and helping to create efficiencies in managing resources.
Automation and clinical chemistry
 Nitin Srivastava
Nitin Srivastava
Associate Vice President,
Vector Biotek Pvt. Ltd.
(A Beacon Group Company)
For many people, pathological testing is an unseen side of medical practice. However, most of the clinical conclusions doctors make are based on pathology test results.
Clinical chemistry which is the most imperative discipline of pathological testing today has its roots since early days of 1852, when the Beer-Lambert law was made fully operational, and based on which the first colorimeter was invented in 1870 by Jules Dubesco.
As time progresses, the need for clinical chemistry gained significant importance, and this necessity initiated the discovery of semi-automation of clinical chemistry testing with manual involvement in preanalytical and analytical steps.
The introduction of Robot Chemist in 1959 gave solid foundation to automation in clinical chemistry, which reduces the testing time and enhances the accuracy of testing. With automation, the speed of testing with dependable accuracy opened a whole new era.
The era of automation arrived with the introduction of the autoanalyzer, using continuous flow analysis and the Robot Chemist that automated the traditional manual analytical steps.
Successive generations of stand-alone analyzers increased analytical speed, offered the ability to test high volumes of patient specimens, and provided large assay menus. Development of integrated systems greatly improved the analytical phase of testing. Automation is developed for pre-analytical and post-analytical procedures. All phases of testing were ultimately combined in total laboratory automation through which all modules are physically linked by track system, moving samples from beginning to end. Another important face of automation is informatics, which interfaces the analyzer software to a laboratory information system (LIS) or the hospital information system (HIS). This software includes control of the overall operation of a TLA configuration and combines analytical results with the patient’s demographic information to provide additional clinically useful information.
After successfully addressing the need for mid-size clinical chemistry automation, Beacon Group has recently introduced one more fully automated biochemistry analyzer UNICORN 480 with a speed of 400 tests/hour. This system is an ideal example of perfect balance of speed with accuracy, incorporating many unique features to match the requirement of high-volume testing needs by laboratories and hospitals.
Biochemistry has evolved greatly over time, driven by numerous factors – not the least being technological advancements in the world at large. Computers, microprocessors, and robotics paved the way for automation and cloud-based technology. With this, laboratories no longer consist of small standalone, manually operated units that performed a handful of tests; instead, they have transformed into bustling hubs featuring large integrated platforms that produce thousands of tests per hour with sophisticated information management systems.
In modern times, automated testing systems have become increasingly popular, allowing users to transform their output amongst other perceived benefits, such as less labor required, decreased preparation time, and fewer input errors. Ultimately, the primary difference between automatic and manual systems is that in a manual system, a human tester is required on site to provide the input for processes to run successfully.
Automating chemistry is not actually that new. It is something that people have been trying to do since the early 90s. But the last decade has seen an explosion in its use and in tools commercially available to carry it out. Now, it is pivotal for anyone in order to stay competitive in research and development and quality control.
Automation can be applied to almost any type of chemistry at any scale, from automating reaction conditions to using robots to manipulate reagents and carry out complete reactions.
For medicinal chemists, the ultimate aim is to automate an entire workflow from design and synthesis through to assaying and analyzing – sometimes called the DMTA cycle (design, make, test, and analyze). It is quite a challenge, but that is what they are trying to do. If one can take (the process) from 90 days down to 20 days, then that is a four-fold increase in the number of candidates they can screen.
Speed is not the only advantage that automation provides. One gets increased productivity, increased yields, increased product purity, decreased rejects, decreased waste, and disposal costs. Automating reactions can increase safety by allowing greater control of reaction conditions. Plus, automated processes are also more reproducible.
Automation is unlikely to work for all biochemistry though handling solids is still pretty difficult. A Grignard reaction, for example, uses powdered magnesium, which is not a very safe thing to use.
Even where automation is possible, not everyone is sold on its advantages. There seems to be a fear that automation will not only supplant the role of the bench chemist but will also change the culture of everyday lab science.
 Babli Dhaliwal
Babli Dhaliwal
CTO,
Trident Diagnostics.
Only a couple of years back, biochemistry diagnostic industry was focusing on upgrading the routine and immunoassay analyzers in terms of throughput and stability of reagents, calibration, and control materials. The pandemic in the past two years or more has taken a paradigm shift, and efforts are now focused on molecular biology and genetic testing for diagnosis and follow up of infectious and genetic diseases. Research and resources are directed at better instrumentation and reagents, which use PCR and sequencing techniques. Even in the routine, workload has increased for rare tests like D-Dimer, CRP, immunoglobulins and infection markers. Even established manufacturers of clinical biochemistry are now focusing on the PCR and genome sequencing segment. However, once the pandemic is over biochemistry instruments and reagents will get center stage. Research and development will be on newer tests to assess the immunity levels and fitness assessment.
Undoubtedly, automation will change the role of the biochemist, but perhaps that will be a good thing. It will free up chemists, who typically do really laborious repetitive work, to actually think about the science behind it. The remaining time chemists spend in the lab and in the office is likely to shift further toward the office, with chemists becoming information workers.
There may be less need for technician roles that are heavily based on repetitive laboratory work, but today most technicians are already highly knowledgeable specialists, and it is more likely that automation will create a whole host of new roles for maintaining and running automated systems.
While robots may not be coming for the biochemist’s job just yet, it may be a different matter once automation is coupled with advanced artificial intelligence (AI). In may be 10–20 years’ time, one could see that there will be models with AI, where specialists basically send a request to a web server, that web server runs the chemistry that they want it to look at, and they get a result back. And it is all done in a fully automated way in a few hours.
One thing that is for certain is that automation continues to be the future of biochemistry testing. Those within the science industry are constantly seeking to further innovate new technologies to improve productivity and efficiency within laboratories that strive to produce accurate results as fast as possible. Furthermore, as technology develops and fresh ideas enter the market, the automation of today will likely filter down into smaller laboratories, thus continuing the growth of an automated world of testing.
Perhaps the most common assumption regarding the future of testing within the laboratory is the birth of robotic lab assistants (robots). This shift began at the start of the millennium, with robots being introduced to laboratory settings to carry out previously manual tasks, such as manipulating samples, applying chemicals that aid the process of breaking up DNA into measurable segments, analyzing results, and feeding these results into a computer.
A study by the University of Liverpool in 2020, where a robotic lab assistant machine was created, found that a robot operated approximately 1000 times faster than a human over the course of a working week. The robot was in operation for 22 hours on each of the seven days, something that is simply not feasible when using human input without the uptake of high labor costs. Although robotic assistants create a common fear that there will no longer be a requirement for any human input, this is not true. The machine created by the University of Liverpool required programming and could not physically set up its own experiments. This development will act as a major benefit within laboratories in the future, as while the robotic assistant carries out the tedious, time-consuming tasks, scientists will have more time to carry out new research, which would not be possible in the present time.
As new automated technology becomes available in the future, it is likely that one will see a trickle-down effect with systems becoming more accessible and affordable to laboratories that differ in size and budget. In the past, limited supply and high costs meant that automated technology was often reserved for the benefit of the best-funded laboratories around the globe. However, as the demand for automated machinery continues long term, an increase in supply – in addition to the larger laboratories seeking the latest innovations – will result in an increasingly wide range of automated testing across smaller laboratories, medical centers, schools, and universities. As this occurs, the number of laboratories depending solely on manual testing will further decrease, continuing the automated revolution to clinical testing.
Whatever the future holds, automation will be a part of it in some way, shape or form, so the industry should be looking to embrace it and the myriad of possibilities that come along with it.












