Hematology Instruments and Reagents
Hematology market continues to show steady growth
 The cellular and morphological analysis is an integral part of any hematology laboratory. The hematology analyzers are being used predominantly for cell counts and differential leukocyte analysis. In addition, these analyzers are capable of reporting many additional parameters and can provide much more information than what they are intended to provide, but they are not being optimally utilized to their potential. Automation of cell counting and characterization has revolutionized the results of hematology analysis since its initial development in the 1960s. Although many detection methods are still in use, optical technology has represented a key innovation in automated hematology analysis since its introduction.
The cellular and morphological analysis is an integral part of any hematology laboratory. The hematology analyzers are being used predominantly for cell counts and differential leukocyte analysis. In addition, these analyzers are capable of reporting many additional parameters and can provide much more information than what they are intended to provide, but they are not being optimally utilized to their potential. Automation of cell counting and characterization has revolutionized the results of hematology analysis since its initial development in the 1960s. Although many detection methods are still in use, optical technology has represented a key innovation in automated hematology analysis since its introduction.
Also, revolutionary advancements in computing, electronics and manufacturing, along with continuous innovation in the areas of biotechnology, fluidics and mechanics, have led to a reduction in the size of analyzers. Progress outside of the field of hematology results in more tools and methods for hematological analysis. A short review of any hematology-focused scientific journal or congress showcases that many papers address combining traditional automated analysis and morphological slide review, with innovative genomics testing, advanced automated imaging processing, and microfluidics. While many of these techniques are at the early stages of development and exist outside of routine core hematology laboratories, it will not be long before they become standard for any laboratory staff technologist.
In the past 2 years, several new products and technologies have emerged on the market, all of which promise to change the traditional understanding of laboratory hematology workflow and standards. New automated systems broaden capabilities beyond traditional methodologies that include cellular analysis combined with manual review. Globally, due to fast-moving product development, small entrepreneurial niche manufacturers and large multinational companies have launched products on the scene to provide unique solutions to evolving customer needs.
New tools and parameters will continue to emerge as the market competition increases and technology evolves. Thus, the traditional concept of laboratory hematology will also evolve – to one leveraging a variety of orthogonal techniques to produce deeper clinical insights faster and at a lower cost than ever before. Indeed, this is an exciting time in the field, and the next 5 years would see seismic changes that promise to bring better patient care and more options for laboratory professionals.
Indian market
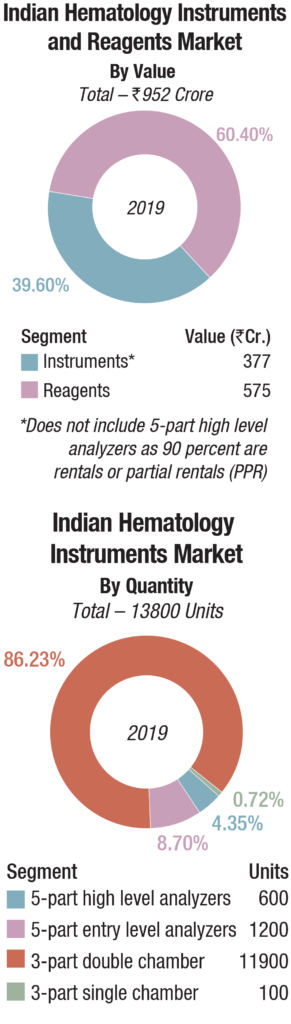
The Indian hematology instruments and reagents market in 2019 is estimated as Rs 952 crore. Reagents constitute 60 percent of the market at Rs 575 crore, and instruments, all fully automated, the balance at Rs 377 crore.
The hematology instruments market may be segmented as 3-part and 5-part analyzers. Within the 3-part analyzer segment, the single-chamber is a dying breed and the laboratories are opting for its double-chamber counterpart, which contributes 86 percent to the total instruments market by volume.
Within the 5-part analyzers, the entry-level or standalone analyzers are the preferred ones and are largely purchased. Within the high level 5-part analyser category, the basic model with an auto loader is more popular. However, the discerning customer opts for the high-end analyser with a throughput of 200-300 samples per hour, that can create four to twelve slides from a single sample aspiration; and 140 slides can be prepared per hour. A handful of buyers also opt for a series of connected hematology workcells, so that the lab can now streamline workflow with smart workload balancing and advanced analytics, providing relevant and thoughtful workflow efficiency, while delivering comprehensive and accurate patient results. The 5-part high level analyzers are almost all placements and are in a very few cases partially placed.
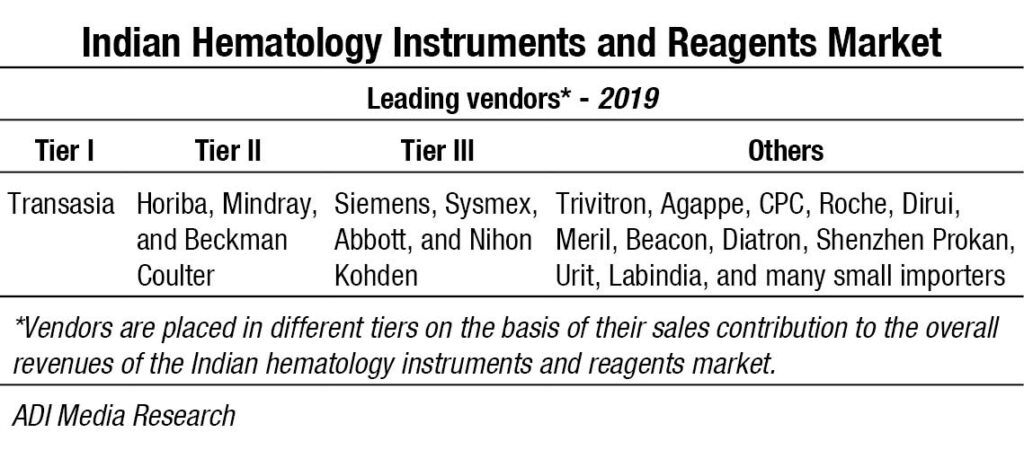 Transasia continues its journey, getting its earlier installations serviced by Sysmex engineers, and making inroads into tier 2 and 3 cities with its competitively priced models. Mindray and Horiba are introducing new products and gradually transitioning to premium customers with a changed basket of fewer standalone systems. Beckman Coulter continues to deliver greater sensitivity through its advanced technology and has a couple of very high-performance models to its credit. Having parted ways with Transasia, Sysmex continues to strengthen its sales and support network. Siemens, Abbott and Nihon Kohden are also aggressive in this segment. Trivitron, Agappe and CPC also provide a complete solution for hematology diagnosis. And many other brands thrive in this segment, catering to regional labs, municipal corporation labs and mohalla clinics and mostly on rental basis.
Transasia continues its journey, getting its earlier installations serviced by Sysmex engineers, and making inroads into tier 2 and 3 cities with its competitively priced models. Mindray and Horiba are introducing new products and gradually transitioning to premium customers with a changed basket of fewer standalone systems. Beckman Coulter continues to deliver greater sensitivity through its advanced technology and has a couple of very high-performance models to its credit. Having parted ways with Transasia, Sysmex continues to strengthen its sales and support network. Siemens, Abbott and Nihon Kohden are also aggressive in this segment. Trivitron, Agappe and CPC also provide a complete solution for hematology diagnosis. And many other brands thrive in this segment, catering to regional labs, municipal corporation labs and mohalla clinics and mostly on rental basis.
With COVID-10 pandemic refusing to relent, most laboratories in the country either remain shut, or are operating at very low output, resulting in minimal purchases of instruments in the March-April-May period. The procurement by government labs also is low. With the recent approval of COVID-19 package of Rs 15,000 crore to build on health infrastructure till March 2024, some expenditure is expected on IgG ELISA assay kits used for the qualitative detection of novel coronavirus infected pneumonia cases, suspected clustering cases, and other new coronaviruses in serum samples through measurement of the COVID-19 IgG antibody. The PCR tests currently used to directly detect the presence of an antigen can be very labour intensive, with several stages at which errors may occur between sampling and analysis. The differential hematology analyzers may help in providing an accurate and precise evaluation and enumeration of the presence of reactive lymphocytes, which play a critical role in management of COVID-19 patients.
Global market
The global hematology analyzers and reagents market is expected to reach USD 9.6 billion by 2023 from USD 7.2 billion in 2019, at a CAGR of 7.4 percent, predicts MarketsandMarkets. The ability of a hematology test to effectively measure several blood components has made it an essential screening tool to diagnose a wide range of blood-related disorders, including anemia, blood cancer, hemorrhagic conditions, and blood infections, among others that affect millions of people each year across all age groups. Increasing incidences of hematology disorders and growing awareness related to the availability of a wide range of diagnostic options to treat these targeted disorders are the primary factors driving the growth of the hematology testing market.
Automation, being the primary focus of the hematology testing, the market has been witnessing considerable technological advancements with respect to products that offer effective, accurate, and fast testing results. The availability of automated hematology analyzers has further led to a reduced administrative error, making the process more effective and efficient in disease diagnosis, as compared to manual hematology testing. Furthermore, factors such as technological advancements in hematology analyzers and reagents and integration of flow cytometry techniques with hematology analyzers are expected to support the growth of this market during 2020-2023.
The market has also witnessed a paradigm shift toward patient-self-testing that eliminates the requirement to travel to a lab, clinic or physicians’ office for having the tests done. With the emergence of high throughput hematology analyzers and point-of-care devices, more companies are expected to enter into the market, investing more in its research and development with an effort to develop cost-effective and advanced hematology systems.
Usage of microfluidics technology in hematology analyzers and introduction of digital imaging system in hematology laboratories could open up opportunities for new players in the global hematology analyzers and reagents market. In addition, increasing focus toward emerging markets, such as India and China, could also open up opportunities for new players in operating in the market. Moreover, safety and quality of hematology analyzer could be a challenge for the growth of the global hematology analyzers and reagents market.
The hematology products and services segment accounted for the largest market share in 2019. This can be attributed to the increasing adoption of PoC testing hematology analyzers in emerging economies, launch of technologically advanced hematology products coupled with the increasing preference toward automation, and rising trend of the reagent rental business model, and the availability of a wide range of services from major hematology providers.
The high-end hematology analyzers segment is expected to grow at the highest rate in 2019. This is attributed to advanced features of high-end analyzers such as reliability, high accuracy, flexibility, ease of use, and multi-parameter testing capability.
North America accounted for the largest share of the global hematology products and services market in 2019, followed by Europe. This is attributed due to factors such as the rising incidence of patients suffering from blood disorders, high number of patients with blood coagulation disorders, growing prevalence of target diseases, and the rising demand for blood transfusions are increasing the demand for hematology testing in North America.
Some major key players operating in global hematology analyzer and reagent market are Sysmex, Danaher, Nihon Kohden, Siemens, Abbott Laboratories, Boule Diagnostics, Horiba, Bio-Rad Laboratories, BioSystems, Diatron, Drew Scientific, EKF Diagnostics, Mindray, Ortho Clinical Diagnostics, Roche, and others.
Vendor update
In February, 2019, Horiba Medical introduced malaria screening and routine hematology. Based on the innovative data mining techniques combined with full blood count, the malaria-suspicion flag is optionally available on both the ABX Pentra XL 80 and Pentra XLR.
In September 2019, Sysmex America, Inc. and LabCorp announced an extension of their hematology automation agreement, to provide LabCorp Diagnostics’ primary reference laboratories with Sysmex latest, upgraded XN-Series technology. The new XN-9100 system is a scalable, modular automation system that incorporates sample sorting robotics from Yaskawa Motoman.
In October 2019, Beckman Coulter announced the commercial launch of its new DxH 690T hematology analyzer. Designed to help laboratories streamline workflow and maximize uptime, the DxH 690T offers all the benefits of the company’s flagship DxH 900 hematology analyzer to mid-size labs, including an industry leading 93 percent first pass yield and the recently released early sepsis indicator. In March 2019, the company has received 510(k) clearance for its DxH 520 hematology analyzer from the US Food and Drug Administration (FDA), and is now available for sale in the United States.
Technology trends
A number of technological solutions have been developed in recent times. These include commercialization of modular, high-throughput, and versatile analyzers, which can be easily interconnected by means of sample conveyers, and can fit the organization of small, medium, and large facilities; integration of pre-analytical workstations, which can be identical to those included in models of total laboratory automation, or can be specifically designed to suit hematological testing; connection with automated slide strainers, which help improving the entire slide-making process (i.e., less manual activities and lower biological risk, improved standardization of slide preparation and staining, customization of staining protocols, reduction of TAT); and integration of automated image analysis systems.
WBC differentials. The automated WBC differential provides the absolute and relative (%) concentrations of the five different types of WBCs (neutrophil, eosinophil and basophil granulocytes, lymphocytes, and monocytes) present in normal blood. This provides information for diagnosis and assessment of infections, immune system or bone marrow disorders, and hemato-oncological diseases. Traditionally, the WBC differential has been determined by manual counting and classification of 100 or 200 WBCs on a stained blood smear. This method, although highly imprecise, is still considered the reference method for the WBC differential, and may be performed as a reflex test after an automated CBC analysis. Today, WBC differentials are usually performed on automated hematology analyzers. Although the automated WBC differential is highly accurate and reliable in the absence of pathological cell types, a manual differential is often required to confirm the presence of immature or reactive cells, blasts, and other pathological cell types.
Impedance and cytochemistry. Electrical impedance is a cell counting and sizing technique based on the measurement of changes in electrical impedance (resistance) produced by a particle (i.e. a blood cell). It was the first automated technology to count cells and measure the size of WBCs, red blood cells (RBCs) and platelets (PLTs). As cells pass through an aperture of known size, they cause a change in electrical conductance which is proportional to the size of the particle.
Impedance technology can deliver a three-part WBC differential, where cells are grouped into three sizes: lymphocytes, mid-range cells and granulocytes, but it does not allow for the differentiation of the granulocyte sub-type. The limitation of this technology is that it is based on purely measuring cell size, so abnormal cells, such as nucleated red blood cells (NRBCs), PLT clumps, giant PLTs or un-lysed red cells, cannot be separated and may interfere with normal cell populations.
Some current hematology analyzers still rely on impedance technology, although with additional technologies to improve the differential white cell count. The utilization of a high frequency signal, defined as RF conductivity, allows the discrimination between different WBC subpopulations.8 RF signals pass through the cell, producing a response that is related to nuclear composition, cytoplasmic density and other differences in internal structures. This technology aims at mitigating the inherent limitations of impedance technology. Cytochemical staining is another technology used to distinguish between cells containing myeloperoxidase (a lysosomal enzyme located in the azurophilic granules of the neutrophils and its precursors, eosinophils, and monocytes) and peroxidase-negative cells, which include lymphocytes and basophils.
Optical technologies. Optical flow cytometry provides several advantages over traditional impedance methods. During optical light scatter measurement, a beam of laser light is passed through a diluted blood specimen stream that is projected into the flow cell by hydrodynamic focusing. As each cell passes through, the focused light is scattered in various directions and detected by photo detectors which convert the signal into an electric pulse. The electronic signals are transmitted to a computer for storage and analysis.
The signals provide information about cellular characteristics such as size, internal complexity, nuclear lobularity/ segmentation and cytoplasmic granularity, which are used to identify the cells. Cells with similar light scatter properties form a cluster in the scattergram and can be separated using advanced software algorithms. Some analyzers only use two angles of light, whereas others use multi-angle optical scatter analysis.
The more detectors (positioned at various angles) that are used to collect signals on each cell, the more information generated on the cellular characteristics, thereby increasing the accuracy of cell identification. The use of optical technology has led to the ability to provide five-part WBC differentiation and depending on the sophistication of the instrument, some current analyzers may report six-part WBC differentials, including immature granulocyte (IG) counts. Adding the capability of detecting a fluorescent signal further enhances the potential of optical technology. Fluorescent flow cytometry captures the light emitted from internal cellular components that are stained with a fluorescent dye as cells pass through the flow cell in front of the laser beam. Fluorescence is frequently integrated with multiple-angle optical light scatter methods to further improve the WBC differential subtype classification.
MAPSS technology. Multi-Angle Polarized Scatter Separation technology uses four light scatter detectors to determine various cellular features. The application of a depolarized light detector is a unique characteristic of this method and allows for specific identification of eosinophil granulocytes. The four detectors generate the following signals:
- 0° or Axial Light Loss (ALL): related to size;
- 0° to 10° Intermediate Angle Scatter (IAS): related to cellular complexity;
- 90° Polarized Side Scatter (PSS): related to nuclear lobularity/segmentation; and
- 90° Depolarized Side Scatter (DSS): related to eosinophil granules.
These signals correlate with morphological characteristics that can be determined visually under the microscope from a stained slide. Various combinations of these four measurements are used to classify the WBC subpopulations and provide morphological flagging. Fluorescent flow cytometry is used to detect NRBCs based on DNA staining when additional reagents and a detector for fluorescence signal is added.
There have been numerous promising directions that could positively impact the future of the automated WBC differential. The International Council for Standardization in Hematology (ICSH) has proposed a new reference method for the leukocyte differential based on the use of monoclonal antibodies (mAbs) and multicolor flow cytometry. This would provide manufacturers with accurate and reproducible WBC classification for the development and improvement of automated technologies.
Another opportunity for improvement is the incorporation of additional light scatter measurements in the characterization of blood cells, with the aim of more accurate classification of WBC subpopulations and potential identification of immature or pathological cell types. For example, advanced MAPSS technology can enable the differentiation of seven subpopulations of nucleated cells including neutrophils, lymphocytes, monocytes, eosinophils, basophils, IGs (including metamyelocytes, myelomyelocytes and promyelocytes), and NRBCs (if present), by utilizing seven light scatter detectors and fluorescent flow cytometry.
Outlook
The many technological advances occurred in laboratory medicine over recent times have enabled the introduction of a vast array of innovations, which have led the way to a more efficient patient care and a more convenient organization of resources and workflows within the laboratory. In the foreseeable future, the better understanding of phenotypic heterogeneity of RBC disorders, also supported by IT tools, such as expert diagnostic systems or artificial neural networks, will predictably enable to improve the global management of these disorders at multiple levels. Yet, some additional issues will need to be addressed, on top of it all the current lack of harmonization in laboratory hematology.
Industry Speak
Hematology instruments and reagents market in India
 Rajesh Patel
Rajesh Patel
Business Head,
Agappe Diagnostics Ltd.
Hematology is one the major segments in the IVD market in India, which is estimated at more than Rs 1000 crore in the year 2019. The segment is controlled by 3-part hematology analyzers and its corresponding reagents with a market share of ~65 percent, and the remaining market is controlled by 5-part hematology analyzers. It is estimated that more than 11,000 units of 3-part hematology analyzers are installed in India in 2019, followed by 3000 units of 5-part hematology analyzers.
Out of the existing more than 55,000 laboratories in India, more than 40,000 laboratories are in the Tier-II and Tier-III segments, covering the rural and semi-urban population with limited access to majority of IVD technologies. As per statistics, more than 70 percent of the Indian population lives in the rural area, and the benefits of IVD technologies remain underserved to the patients in these rural areas. One of the major changes in the hematology segment is the introduction of highly compact 3-part and 5-part hematology systems. These compact systems are well suited for rural India, and are expected to bring in changes in the IVD industry. The penetration of CBC in rural areas will help to assess the prevalence of various anemia (which is one of the major concerns in India) among the rural population and its control, which is an objective of the NRHM, and an affordable and accurate 3-part hematology analyzer is the need of the hour. The hematology market in India is dependent only on import – the major suppliers being from China, Europe, Japan, and USA. The current inflation in the forex rates is one of the major concerns in the Indian hematology segment. The recent pandemic of COVID-19 has created a huge demand in hematology testing where a low WBC count, along with a decreased lymphocyte count, is of importance in the early stages of the disease, further adding to our woes on cost of testing.
Truly indigenous hematology systems are in the pipeline with state-of-the-art technology to suite the Indian scenario, and these are expected to hit the market in the coming months. It is believed that with local manufacturing both the hardware and consumable cost of CBC testing will be reduced drastically, and it will bring down the dependency of our country on imports. This India’s truly first indigenously developed and manufactured 3-part hematology analyzer will change the overall market of hematology, which is long awaited since more than five decades.
Automated hematology – More than CBC analysis
 Dhiren Trambadia
Dhiren Trambadia
Associate Vice President, Diagnostics,
Trivitron Healthcare
Complete blood count (CBC) forms the basis for a number of hematology and pathological diagnosis. Over the past decades, hematology analyzers have experienced great technical advancements, featuring low turnaround time, enhanced precision, and accuracy. The challenges associated with manual methods like immature cells, reliability on results, distribution error, and statistical error are being replaced with precise, safe, and dependable automated hematology systems.
Ever since the initial developments in the 1960s, automation in cellular analysis has revolutionized the results of hematology analysis. Automation in hematology systems offers a platform for more than just CBC testing from a single sample. However, with technological advancements, instruments based on the principles of electrical impedance, optical scattering, flowcytometry, and fluorescence carry out accurate quantitative measurements with higher sensitivity and specificity to mark abnormal cells. Given the availability of various detection methods, optical technology became the most widely employed.
In the recent years, the hematology analyses are included with the research parameters like atypical lymphocytes, large immature cells, immature granulocytes, and N-RBC, which help the pathologists in easy diagnosis. With advancement in the laser scattering technology reticulocytes, immature reticulocyte fractions are also measured.
Further procedure improvements are currently available with pre- and post-analytical sample sorting/documenting and automation of smear review. Integration of newer techniques like microfluidics and digital imaging systems in hematology laboratories are expected to revolutionize the hematology market. Modern hematology analyzers are efficient enough to extract information such as blast-cell differentiation, which provides detailed cellular information to the clinician. This has been made possible with advances in instrumentation and technology evolving together in a cost-conscious environment. Trivitron Healthcare provides a complete solution for hematology diagnosis.
ESR automation –A preview
 Sudipta Acharjee
Sudipta Acharjee
Senior Product Executive,
CPC Diagnostics Pvt. Ltd.
Erythrocyte sedimentation rate (ESR) is the rate at which red blood cells (RBC) sediment in a period of one hour. Though ESR is a non-specific test, it is still widely used in clinical practice as an indicator of inflammation, infection, trauma, or malignant disease. An ESR test is being used in cases of arthritis, vasculitis, or inflammatory bowel disease, and also to monitor the prognosis of the diseases.
ESR, which is still one of the major tests performed in hematology laboratory, remains a manual procedure and one that is associated with a level of subjectivity in interpretation. The current procedure generally uses the gold standard Westergren method, using sodium citrate method, or Wintrobe method by using the EDTA tubes as well, but these methods always pose a potential risk in the form of exposure to biohazardous materials, leading to blood-borne infections to laboratory professionals. These manual methods also lack specificity. One approach of making ESR tests safer, quicker, and precise is the development of automated analyzers.
The modern fully automated ESR analyzers make the procedure simple and effective. Initially, many analyzers were developed based on the Westergren method of using sodium citrate tubes for analysis of ESR; later, in order to make it cost-effective to the laboratory and patient-friendly, analyzers were made to perform the test with EDTA tubes, which is widely accepted and being followed in many of the laboratories.
These ESR analyzers help in avoiding probability of human errors, reduction in the amount of labor needed, and reduction in the turnaround time (TAT) of the laboratory by providing results in minutes/seconds. The automated systems also help us in monitoring the real-time sedimentation of the curve. The automatic mixing process makes it ultimate and reduces the risk of biohazard. The bidirectional LIS makes the smooth flow of data, which helps the laboratory to enjoy the true benefit of automation. All the mentioned advantages make the lab to go for ESR automation.
Emerging trends and AI
 Rahul Singh
Rahul Singh
Product Manager (XP&XN-L series),
Sysmex India
The uncertainty in predicting what the future holds for blood cell counting can be best visualized by thinking how difficult it would have been to predict the present situation 50 years ago! However, there will be the dichotomy of building better cell counters for improved diagnostics versus smaller, more portable, or even wearable or implantable in-vivo devices. Such implantable or wearable devices can predict the disease status by monitoring hematological parameters in real time.
Artificial intelligence (AI) is the use of computer algorithms to perform tasks or solve problems that were traditionally solved using the adaptable and flexible human intelligence. Indeed, as of 2020, radio-diagnostics is the field where AI has made the most progress to date; 90 percent of the practicing radiologists anticipate that AI will be incorporated in their future practices. AI has also been employed in the analysis of hematopathology data for better informed diagnosis, prognosis, and treatment planning. The foundation knowledge related to benign and malignant hematology is also evolving with the application of AI in hematology. Even the most skilled hematology specialist can overlook the patterns, deviations, and relations among the vast number of blood parameter measures by modern analyzers. In contrast, the AI algorithm can easily analyze hundreds of attributes (parameters) simultaneously with correlation, and recognize a pattern or fingerprint of certain hematological abnormality. The AI algorithms of modern hematology analyzers are trained on sufficiently large datasets of clinical cases that include laboratory blood tests, performed and confirmed by hematology specialist using various confirmatory tests. The machine learning (ML) algorithms can uncover the disease-related patterns in a blood test that may be beyond medical knowledge, resulting in higher diagnostic accuracy as compared to traditional quantitative interpretation, based only over reference ranges. Also, it can lead to a fundamental change in differential diagnosis and result in the modifications in the presently accepted guidelines.
Recently, researchers thave successfully developed and tested a deep learning-based algorithm – convolutional neural networks. This system is applied for differentiating myelodysplastic syndrome from aplastic anemia with 96.2 percent of sensitivity and 100 percent specificity. This is the first such system for MDS, using peripheral blood smears; it can further be developed for automated diagnosis of various hematological abnormalities. This system recognizes over 100 patterns in cell size and cytoplasmic morphological features for detection and classification of myeloid malignant cells including MDS. Further training of this AI algorithm can improve the accuracy and reproducibility, which may surpass the human eye.
An understanding of AI’s basics will need to become a part of physician’s statistical literacy in the very near future. AI and ML algorithms and their application in hematology show a great promise in medical laboratory diagnoses. It will be of high value not only for laboratory physicians and patients, but will also have widespread beneficial impacts on healthcare costs!
Hematology – Vital role in patient diagnosis
 Nitin Srivastava
Nitin Srivastava
National Sales Manager-IVD,
Nihon Kohden India Pvt. Ltd.
In the past few years, the IVD market is growing expeditiously and it is an important segment of the global healthcare industry. This growth is fueled by technological advancements, better diagnostic tools, improved treatment monitoring, and increased availability of over-the-counter tests. Technological advancement in high-throughput hematology analyzers, integration of basic flow cytometry techniques in modern hematology analyzers and developments in the high-sensitivity point-of-care (PoC) hematology testing are some of the key factors that are fueling the growth of the hematology market.
In the hematology segment, CBCs contribute to the general assessment of health for patients during regular check-ups. Conditions like anemia, other blood disorders, infections, allergy, and iron and vitamin deficiencies are easily diagnosed through a CBC. Hematology is also used to monitor patient health and alert medical professionals to diseases requiring diagnosis through additional testing or changes to ongoing treatment. Clinical hematology leverages various IVD technologies, including blood analysis, flow cytometry, immunodiagnostics, molecular diagnostics, hemostasis or coagulation testing, histology, and cytology. Now technological advances are being incorporated into hematology analyzers, and through a simple routine CBC with differential, it allows the laboratories to gain access more cellular information than was ever available before. This new information is used to improve cellular counting and decrease the rate of unnecessary manual reviews. These technologies, while often invisible to the operator, can translate into a smoother workflow for the laboratory and an improved diagnostic quality of care for patients.
Despite a high number of automated blood cell counters with latest technology in use, a significant percentage of samples require a manual cell differential count of blood specimens under a microscope. The detection of abnormal cells on the basis of complex morphology, especially the size and shape of abnormal cells like immature white blood cells or diseased cells. It is important, though, to accurately detect the presence of immature white blood cells since they are often associated with conditions such as leukemia, infection, inflammation, or tissue injury. Accordingly, manufacturers are working to decrease the number of manual reviews that are required, through improvements in analyzer accuracy in differential count.
Hematology, a long way to go
 Nidhi Joshi
Nidhi Joshi
CEO,
Biogeny Diagnostics Pvt. Ltd.
Today, the highest level of automation is scalable and configurable, starting from piercing the sample tubes to conducting variety of determinations, CBC, 6-part, WBC differential, NRBC, RET and immature retic fraction (IRF), automated immature platelet fraction (IPF), automated smear preparation, and staining. These tests are standardized assays that meet performance goals, decrease technologist hands-on time, eliminate batch testing, and provide results faster to physicians. Testing together with hematology-specific middleware product, laboratories are reporting up to 85 percent of their test volume without any operator intervention.
In addition to these, hematology automation platforms offer more than CBC testing from a single EDTA sample. Laboratories that have incorporated HbA1c testing on high-speed hematology lines are performing >90 percent of assays with minimal technologist intervention. Auto validation of HbA1c results can run as high as 90 percent. Further process improvements are coming to the forefront of hematology, such as automation of digital smear review. Now, these newly formed work areas can manage traditional hematology testing as well as the HbA1c, traditionally tested in the chemistry department.
One area of hematology testing is reagent preparation. Hematology technologists frequently required to change 20 litres cubes of diluent. Recent additions to automated hematology lines address this concern by including units that utilize concentrated reagent, which is diluted using an in-lab water supply. This approach minimizes the time and effort required to prep the analyzers prior to the highest volume run of the day, and enables laboratorians to avoid interruptions in testing due to the need to frequently change the diluent in high-volume testing settings. Added to these innovations, the work in the direction of pre- and post-analytical sample sorting and archiving, effectively helps in minimizing errors in pre- and post-analytical work.
Opportunities in these areas are enormous, and will surely go a long way in coming years.
Backed by years of expertise in IVD manufacturing, Beacon Group has recognized the significance of generic hematology reagents, and in 2009 the company started a specialized division – Biogeny. The major initial business concept of Biogeny was to provide generic hematology reagents for the wide variety of 3-part differential analyzers available in the market. These reagents, which are manufactured at one of the most ultramodern plants of Biogeny, meet with the ongoing demand of the market for giving quality results with economy, and this has opened up a whole new area of business in Indian IVD market. Today, in the area of generic hematology reagent business, Bonavera brand has almost become a synonymous name and we as a company are continuing to give more improved versions of products and services to remain as the top-class leader in this product category.
Evaluating hematology analyzers
 Priyank Dubey
Priyank Dubey
Marketing Manager,
Mindray Medical India Pvt. Ltd.
Hematology analyzers have seen tremendous transformation in the last decade; the new technology, data sciences, and integration have opened up many avenues. From increasing amount of information available from hematology analyzers to AI and software tools have enhanced accessibility, flexibility, and advantages offered by integrated systems.
The plethora of information and the features available make the job of selecting the right analyzer quite daunting. One of the parameters, which can assist a laboratory in making the correct decision is efficiency. Mathematically, the quantity of output generated to the amount of input is generally referred to as efficiency, though in this context the trick is to define the right inputs and the desired output from hematology analyzer, as per the laboratory.
The key considerations in the form of output from hematology analyzers, relevant for a high-workload lab, are throughput of the analyzer, number of reportable parameters (normal+abnormal), number of relevant research parameters, sensitivity and specificity of flagging algorithm, visual patterns to assist interpretation, and a decision-making software, which can integrate all this information at a single screen and make it accessible remotely.
As compared to outputs, inputs can more easily be defined in terms of cost-per-test or cost-per-test reportable, depending on the laboratory’s own practices. So, once you have defined the key outputs and inputs from the laboratory, the next step is to assign weights to the output, per unit input, and derive an evaluation factor to assist selection.
Additionally, several factors that are noteworthy in selecting the systems are: any extra/additional charges in terms of license fee for different modes, availability of dedicated body fluids mode, flexibility in terms of disabling unrequired modes, use of most modern technology like fluorescence, service and application expertise and availability, and overall presence of the system in setups of similar structure.
Mindray’s CAL system CAL 6000 and CAL 8000 are fully automated integrated systems that have gained popularity in recent times, where many modern laboratories have chosen CAL systems for up-gradation. CAL system provides the best-in-class efficiency, with the highest throughput of 200 samples per hour from a single system, which can be further increased to 800 samples per hour, 37 reportable, and 48 research parameters, utilizing unique 3D technology, providing better classification of normal and abnormal cells, availability of an automated slide stainer, dedicated body fluids mode, and the LabXpert software, which acts as the brain of the system, making all critical and relevant information available along with the analyzer, and remotely through its unique multi-installation capabilities, thus, providing a holistic solution to the laboratory looking for a high-efficiency hematology analyzer.
Second Opinion
Procurement planning in a hematology lab
 Dr Neeraj Jain
Dr Neeraj Jain
Managing Director,
Jain Diagnostics
Unlike pharmaceutical products, methods for selection and procurement of diagnostic analyzers in hematology labs have to be tailored to the end user. Hence, procedures may differ between the types and levels of labs. In particular, the desired testing throughput, staff competencies and training requirements, and site infrastructure will greatly impact the choice of analyzers in a particular health facility. In addition to national policies and guidelines, financial and regulatory factors may impact a lab’s capacity to select and manage particular types of diagnostic analyzers. Disease patterns that are national, regional, and seasonal may dictate the required scope of services and throughput in specific locales. At facility level, facility infrastructure including stability of utilities, cold chain and/or appropriate storage, human resources capacity, staff competencies, training, local service and technical support, test through-put, and quality-assurance measures will all contribute to product selection and, therefore, procurement of analyzers.
All organizations, whether governmental, non-governmental, or private, have limited resources that must be utilized in the most efficient means possible. The importance of advance planning must never be underestimated as it provides critical information management required to allocate resources that will enable the organization to reach its objectives. The procurement-planning process can be described in several stages ranging from a preliminary-needs assessment through key communication and decision-making stages to the actual development of a written plan. The final stages include the selection and quantification of products. Planning is an iterative process that requires continuous review and inputs from key stakeholders, good communication between parties, and flexibility in approach.
Minimum acceptable performance criteria must be agreed and include the following characteristics, as appropriate for the particular product category: clinical sensitivity, analytical sensitivity, clinical specificity, invalid rate/non-reportable results, inter-reader variability, if subjectively read, and bias for quantitative method. The operational aspects of diagnostics are equally as important as the performance characteristics, and thus both must be included within product specifications. Quantification is the process by which requirements for laboratory equipment, reagents, consumables, and durables are made. The quantification process requires significant preparation to ascertain the current scope and demand for products. A quantification process is dedicated to development.
Forecasting is a projection of the quantities of products required to meet demand for a future period of time. Forecasts are most often made for a 1–2 year period. The lack of available high-quality demand data contributes greatly to low-quality forecasts and resultant stock-outs and wastage of supplies. To conclude, procurement planning is very critical in a lab set-up and requires meticulous planning and implementation for the desired output.
The hematology analyzer – A real work horse
Dr Su nita Deshmukh
nita Deshmukh
HOD – Dept of Lab Sciences & Blood Bank,
Paras Hospitals
Complete blood count is the basic test, required to evaluate a patient’s general health status. Modern analyzers are compact, sturdy, high-end, and high-volume processing machines. These are available as 3, 5, 6, and 7-parts differential analyzers suitable for low-volume to high-volume work load in laboratories.
The analyzer scans 18–50 parameters including clinical and research parameters. Automatic smear preparation and staining is also available with the high-end models. Hematology market in India has yielded approximately Rs 1500 crore in 2019. Hematology instruments’ installation base in India today is around 100,000 3PDAs and around 20,000 5PDAs, and it increased by approximately 12,000 3PDAs and 1000 5PDAs in the last year.
Three-part analyzers provide sufficient information in most clinical settings and are cheaper, whereas accuracy and speed of testing is improved with five-part analyzers. The reagents are available with sufficiently long shelf life and on-board stability. Apart from the volume of reagents used in testing, costing is also dependent on volume of reagents consumed in various cycles like start-up, sleep-wake up, shut down, and frequency of controls and calibration.
In terms of technology, electrical impedance has firm foothold on determining overall number and size of cells, and flowcytometry has proven its worth in differentiating WBCs and identifying abnormal cells. But emergence of optical counting and use of fluorescence for advanced parameters have proved to be a boon to hematology practice.
Special parameters on high-end models include reticulocyte count with hemoglobin content within (RetHe) – sensitive indicator of iron-deficient erythropoiesis, immature platelet fraction (IPF) – and offers guidance on the etiology of thrombocytopenia, and helps anticipate bone-marrow and platelet recovery; early sepsis indicator – new emerging parameter. By utilizing this indicator in the sepsis-management strategy, early treatment of patients leading to reduced sepsis cost burden is anticipated; body fluid cells and NRBC counting – leading to reduced laborious and time-consuming manual processing and inter-observer variability; and detection of hemoparasites (malaria).
Hematology analyzers are the real work horses of clinical laboratories. The advent of digital image-based cell counting and application of artificial intelligence will add more value to patient care, but microscopic examination by experienced personnel is still the gold standard in diagnosis of hematological abnormalities.












