HPLC Systems
HPLC, a panacea of the future
HPLC is certainly going places, and the importance of the discipline cannot be understated.
High-performance liquid chromatography is the largest product segment in the analytical instruments industry. The versatile and popular technology helps identify and analyze constituent components of various chemicals and materials, and is thus used to analyze substances in a wide variety of industries for R&D purposes, quality control, and process-engineering applications. Currently, pharmaceutical with a market share of over 55 percent, and life science industry accounting for more than 17 percent, are the biggest application areas for HPLC testing.
The HPLC market is expected to be driven by new applications such as food safety testing as HPLC is ideally suited for testing of food contaminants owing to its large volume of data generation through a single analysis, thereby facilitating screening, confirmation, and quantification of all the constituents. Proliferation of new applications, especially in biotechnology and pharmaceutical sectors, represents another major factor driving growth.
The HPLC devices are now capable of analyzing almost all types of biological compounds including small molecules that can be isolated or synthesized. HPLC is also being used in the purification of peptide therapeutics, as well as certain proteins. Continued improvement in the system automation and in robotics is also one of the major factors propelling growth in the HPLC market. Emerging hyphenated methods such as GC/LCMS and LC/HPLC-NMR represent a shift toward multidimensional hyphenated systems for highly advanced research and quality monitoring applications.
Indian market
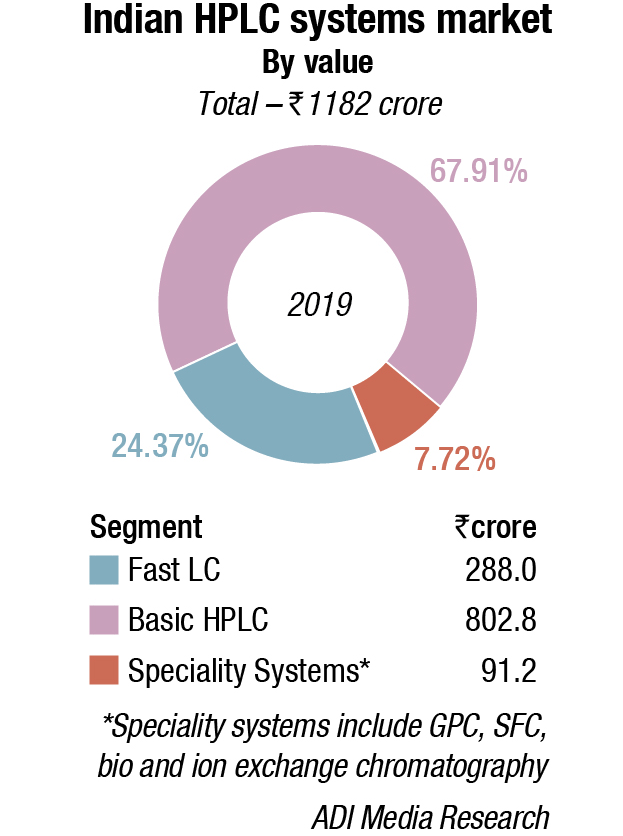
The Indian HPLC market is estimated at `1182 crore, with 5500 units. The belly of the market continues to be the conventional, basic models with an 81-percent share by volume and 68-percent share by value in 2019. The vendors expect the market to increase at 7–8 percent per annum over the next three years, as compared to 12 percent earlier.
 Shimadzu for the last couple of years has had success with aggressive pricing, newer introductions, and a longer warranty period. The company particularly had good sales in the last two quarters of FY21. Of course, Shimadzu’s focus is more on the QC and QA labs, whereas Waters and Agilent products find more uses with the discerning customers at research and innovation labs.
Shimadzu for the last couple of years has had success with aggressive pricing, newer introductions, and a longer warranty period. The company particularly had good sales in the last two quarters of FY21. Of course, Shimadzu’s focus is more on the QC and QA labs, whereas Waters and Agilent products find more uses with the discerning customers at research and innovation labs.
HPLC is commonly used for the separation and analysis of non-volatile compounds. Due to the flexibility of this technique, numerous applications have been adopted for routine use in the pharmaceutical industry. The industry received a slight setback from the Indian companies as there was a significant increase in the US Food and Drug Administration (USFDA) scrutiny in 2019. USFDA issued 19 warning letters to Indian companies in 2019, compared to 11 in 2018. Further escalations or delays in resolution would impact the US sales of Indian companies.
At a time when supply-chain disruptions due to COVID-19 pandemic are causing drug shortages across the world, four of India’s drug companies have recently had their manufacturing facilities cleared by the USFDA. These include Lupin’s manufacturing facility in Nagpur, Dr Reddy’s Laboratories’ plant 5 at Miryalaguda in Telangana, Strides Pharma’s plant at Bengaluru, and Biocon’s insulin plant in Malaysia. For large Indian generic drug makers, regulatory hurdles in the US had led to revenue erosion and subsequently a fall in the market value – Indian pharma companies have taken a hit worth nearly USD 1 billion in their market cap in the past 12 months, hence impacting their procurement decisions.
Moving forward, there seems to be a big opportunity in pharma manufacturing as the US, South Korea, and Japan are preparing their exit strategy from China. While Japan would prefer India for its exports of formulations and active pharmaceutical ingredients (APIs) as India has sound expertise to ensure its products and manufacturing adhere to US and EU regulations, Vietnam and Taiwan will be Japan’s choice for its domestic drug consumption needs. Japan and South East Asia will also see Indian pharma companies as their choice since it provides an entry point into the European Union and the US markets.
Global market
The global HPLC market size is projected to reach USD 5.7 billion by 2025 from USD 4.5 billion in 2020, at a CAGR of 4.6 percent. The growth in this market is driven by high sensitivity and accuracy of the HPLC technique, the rising importance of HPLC tests in drug approvals, the growing popularity of hyphenated techniques, and increasing pharmaceutical R&D spending. However, the high cost of HPLC systems is expected to restrict market growth to a certain extent.
Adoption of HPLC instruments is expected to increase. The rise in pharmaceutical, biotech, food, and agriculture research and development is sure to propel the market. Patented studies and growth in clinical trials will surely boost the market. The consumables market is also expected to rise at the highest CAGR over the next 5 years.
The clinical research segment will grow at the highest rate. The HPLC instrument is critical for clinical studies and research. It is required to determine inorganic impurities such as plant or animal proteins existing in pharmaceutical bulk materials or excipients or catalysts. It is also used in the testing of organic compounds. Clinical research involves evaluation studies of the developed drug. This calls for efficient analytical testing instruments. Thus, a large market share of this application is witnessed, which is followed by the use of HPLC in other applications (analysis of cosmetics, environmental studies, and food industry).
North America market is projected to witness the highest growth rate. North America is expected to account for the largest share of the global HPLC market. Market growth in this region is driven primarily by the increase in the funding for R&D, a growing number of preclinical activities by CROs and pharmaceutical companies, and the increasing food and agricultural industry in Canada. On the other hand, Asia-Pacific is projected to have the highest growth rate during 2020–2025. The high growth rate of the Asian region can be attributed to factors like extensive sales of biosimilars and generics in Japan, and the growth in the pharma and biotech sectors in India and China.
The global HPLC market is well established owing to the dominance of prominent market players like Waters, Agilent, Shimadzu, Thermo Fisher, PerkinElmer, GE Healthcare, Bio-Rad, Merck Millipore, Hitachi, Showa Denko, Gilson, Phenomenex, Jasco, Hamilton Company, SIELC, Orochem, YMC Co. Ltd., Restek, Trajan Scientific, and Tosoh Bioscience LLC.
Increasing sample throughput
Using simple first principles or free software tools, it is possible to significantly increase sample throughput and reduce solvent consumption for many legacy HPLC methods providing a boost to lab capacity and speed of data output. The magnitude of the improvement will depend upon the laboratory instrumentation available; UHPLC offers impressive numbers but modified rapid HPLC options are still worthy of consideration. This is especially pertinent in high sample volume or time-critical environments such as in-process testing, clinical, forensic, or doping laboratories.
It is possible to obtain significant reductions in method run times by quantitatively translating the method to a shorter length column with smaller particles (either fully porous or solid core). The principle for increasing sample throughput, whilst maintaining the original method performance, is to ensure the column length (L) to particle size (dp) ratio (L/dp) is kept consistent. This results in similar separation performance being observed in a reduced time. Software LC method translation tools (e.g., the ACE Translation Tool) include all the necessary equations to accurately scale method parameters, including injection volume, flow rate, gradient profile, etc., and can be downloaded for free.
Significant improvements in throughput can be realized, without sacrificing method performance and/or robustness. In addition to reducing analysis time, compelling reductions in solvent consumption are also achievable. The translation of methods to shorter columns is often discussed in the context of migrating methods to UHPLC; however, impressive improvements can also be realized using standard HPLC instrumentation, thus improving the utilization of existing equipm ent platforms.
Improving sample throughput for isocratic LC methods. The original separation, using a 150×4.6, 5 µm column has a run time of 35 minutes, with a back pressure of 66 bar. By translating the method to a UHPLC column (50×3.0 mm, 1.7 µm) on a UHPLC instrument, the run time is reduced to 4 minutes with a moderate pressure of 558 bar. This equates to a >8 times increase in sample throughput and a >88 percent reduction in the runtime. However, if UHPLC instrumentation is not available, sample throughput could still be more than doubled by using the existing HPLC instrument with a shorter HPLC column and smaller particle size (100×3.0 mm, 3 µm). In this case, sample throughput is >2 times better than the original method with run time reduced by 60 percent to 14 minutes at a reasonable 215 bar. From a solvent perspective, analyzing 100 samples (excluding equilibration times, cleaning, and shutdown methods) would require 3500 mL with the original method, 500 mL for the UHPLC method, and 994 mL for the modified rapid HPLC option.

Improving sample throughput for gradient LC methods. Using a 150×4.6 mm, 5 µm column, shows a gradient analysis of non-steroidal anti-inflammatory drugs. The post-gradient re-equilibration time from the gradient table is 20 minutes (or ~13 column volumes). It is possible to translate the gradient method to a new UHPLC format, or a modified rapid HPLC format to understand the impact on sample throughput. Using similar principles, along with a software translation tool, it is possible to quantitatively translate the gradient method to the two new column formats. For gradient methods, it is also necessary to scale the gradient profile and correct for differences in instrument dwell volume to ensure the same gradient separation and resolution is obtained with the new column formats. Table 1 shows the original and recalculated gradient times for the separation on each column format.
 The original HPLC separation, using a 150×4.6 mm, 5 µm column, has a run time of 34 minutes, but a total cycle time of 54 minutes owing to gradient re-equilibration, with PMAX of 64 bar. Translating this to the UHPLC format gives a 3.6-minute run time and total method cycle time of 5.7 minutes with PMAX of 510 bar.
The original HPLC separation, using a 150×4.6 mm, 5 µm column, has a run time of 34 minutes, but a total cycle time of 54 minutes owing to gradient re-equilibration, with PMAX of 64 bar. Translating this to the UHPLC format gives a 3.6-minute run time and total method cycle time of 5.7 minutes with PMAX of 510 bar.
This provides a >9 times increase in sample throughput and a >89 percent reduction in runtime/cycle time. The modified rapid HPLC format data, has a new run time of 13.4 minutes and a total cycle time of 21.3 minutes (including equilibration) with PMAX of 193 bar.
This represents a >2.5 times increase in sample throughput and a >60 percent reduction in runtime/cycle time. Solvent consumption for the total cycle times for each format (but excluding initial equilibration and shutdown methods) for 100 samples can be calculated as 5400 mL, 718 mL, and 1515 mL for the original, UHPLC, and modified rapid HPLC methods respectively.
The takeaway
HPLC is certainly going places, and the importance of the discipline cannot be understated.
By making key breakthroughs, HPLC enables the development of various other fields that deploy its tools and equipment. As technology continues to progress exponentially, one can expect to see a spate of new discoveries in the coming years.
Second Opinion
Role of HPLC in clinical laboratory
Dr Aarti Khanna Nagpal
Section Head – Hematology,
Clinical Reference LabHPLC is a form of column chromatography, used to separate, identify, and quantify each component in a mixture. In clinical laboratories, this technology is widely used in HbA1c measurement and detection of hemoglobinopathies. HbA1c represents the fraction of hemoglobin bound to glucose, and presence of a coincidental hemoglobinopathy can affect A1c measurement in various ways, and this depends on the method used for detection. The Bio-Rad Variant II Turbo, installed across SRL reference labs, is a fully automated ion-exchange HPLC system for mid- to high-volume laboratories, which provides a comprehensive solution for HbA1c testing; it delivers quality results in 97 seconds. There is no known interference in the presence of Hemoglobin S, C, D, or E traits and HbF concentrations up to 25 percent. This method is NGSP-certified and traceable to diabetes control and complications trial. It provides rapid separation without compromising the level of resolution of the HbA1c peak from potential interfering compounds, i.e., labile HbA1c and carbamylated HbA1c.
HPLC has also proved to be an accurate and simple technique for quantitation of HbA2, HbF, and other hemoglobin subtypes. The Bio-Rad Varant II Beta thalassemia short program is being used at SRL Laboratories for the presumptive identification of many abnormal hemoglobin variants. This method is capable of separating more than 45 commonly encountered hemoglobin variants within 6.5 min, based on the characteristic retention time. The simplicity of the sample preparation, superior resolution of the method, and accurate quantitation of hemoglobin concentration, combined with complete automation, makes this an ideal methodology for the routine diagnosis of hemoglobin disorders in a clinical laboratory. Both percentages of HbA2 and HBF are calibrated for the highest levels of precision and accuracy. Each chromatogram shows peaks of Hb A0, A2, and HbF along with C window, D window and S window. Other relevant tests like family studies and molecular testing are required in a few cases.
Nowadays, HPLC is a very valuable tool in the clinical laboratory. In the future, more and more HPLC methods will replace old photometric assays and also immunoassays, since these methods fulfill the claims in clinical chemistry – high precision, accuracy, sensitivity, and specificity.
Evolving trends of HPLC
Dr Shaloo Kapoor
Chief Pathologist,
SRL, Fortis Escorts Heart InstituteHigh-performance liquid chromatography (HPLC), also known as high-pressure liquid chromatography, is an advanced type of liquid chromatography, and is evolving ever since Russian scientist Mikhail Tsvet invented the chromatographic technology. The high pressure makes HPLC much faster than gravity-based systems, while still maintaining high resolution. HPLC is routinely used to measure therapeutic drug levels as well as the presence of drugs of abuse, analysis of catecholamines, and in determination of amino acid metabolism. HPLC is an automated, highly reproducible, rapid platform used for HBA1C estimation and detecting hemoglobin variants for screening of hemoglobinopathies as well as providing precise measurement of HbA2 and HbF. It is also used for porphyrins speciation in urine and estimation of various vitamins and biomarkers in diagnostic labs.
Recent trends
HPLC has been recently challenged by ultra-high-performance liquid chromatography platform, which on switching over provided improved separation efficiencies, faster mobile phase, and better chromatographic resolution without disadvantages of high pressure. The analytical technique, liquid chromatography-mass spectrometry (LC-MS) is complex as it combines the physical separation properties of liquid chromatography (or HPLC) with the mass properties of MS (mass spectrometry) with widespread adoption. With implementation of more sophisticated technologies like more sensitive detectors, HPLC systems are incorporating new modules using dual pump to offer three workflows that increase productivity in a single system – dual LC, tandem LC, or LC-MS mass spectrometry. The benefits of chromatography-based technique integration with MS include a simple, rapid technique for analytes, offering high sensitivity and selectivity without pre- or post-column derivatization. Decisive for the success of LC-MS will be the automation of sample preparation, reliability of process, analyzers being user-friendly and their smooth interfacing with LIS. Currently, lot of research is being done on seamless integration of droplet microfluidics on a single high-pressure resistant microfluidic glass Lab on a Chip. Technology enhancements in HPLC are likely to increase throughput, gain more confidence in results, and drive down cost per sample.











