HPLC Systems
HPLC Gaining Prominence In The Pharma Industry

The increased use of HPLC in the drug-approval process by the pharmaceutical industry is expected to drive the market in coming years.
The rapid development of high-performance liquid chromatography (HPLC) instrumentation and technology opens numerous possibilities – and entails new questions. Since its inception, HPLC has undergone technological enhancements in all aspects, whether that is improvement in detectors, better accuracy and precision, or new column chemistries. Adoption of mass spectrometers (MS) as detectors has expanded the capabilities of HPLC even further by enabling the discrimination of small mass variants, particularly in biomolecules.
Unlike product sectors where companies grow but only at the expense of the overall market, HPLC is and remains a growth industry where rising tides raise all ships. A recent research by MarketsandMarkets estimated the global HPLC market to reach USD 4.13 billion by 2021, with CAGR of 5.1 percent, over a five-year period, 2016–2021. The key factors driving growth include high sensitivity and accuracy of HPLC techniques, growing popularity of LC-MS techniques, growing importance of HPLC tests in drug approvals, and increasing life science R&D spending. Furthermore, HPLC is gaining prominence in the pharmaceutical industry as it is used in the development of drugs by assisting in identifying the required compounds and raw materials. Researchers extensively use HPLC for both the quantitative and qualitative analysis of drug samples. HPLC is quickly replacing spectroscopic methods and gas chromatography (GC), as companies are using HPLC to develop standards of control for drug formulations. Thus, the increasing use of HPLC in the drug-approval process by the pharmaceutical industry is expected to drive the market.
In the coming years, the HPLC market is expected to witness the highest growth rate in the Asia-Pacific region. The high growth in the region can be attributed to the growth in biomedical and medical research in Japan, strategic expansions by key players in China, increasing government initiatives, and growing pharmaceutical industry in India, and a favorable regulatory scenario in New Zealand and Australia.
Indian market dynamics
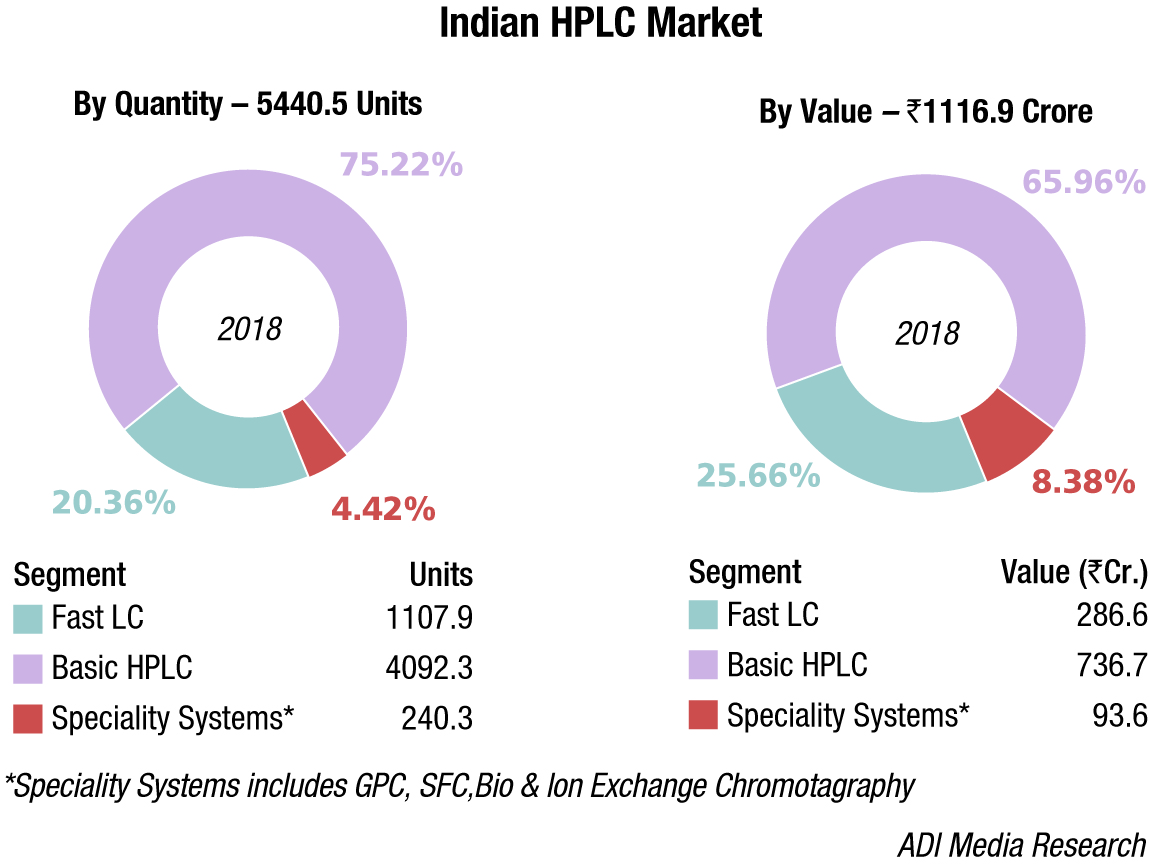
The Indian HPLC systems market saw a decline of 10 percent in 2018, or was at the best flat for some vendors. This may be attributed largely to a dip in the market from non-compliance.
| Tier I | Tier II | Tier III | Others |
|---|---|---|---|
| Waters | Agilent, and Shimadzu | Thermo Fisher (includes Dynex) | Yangling, Perkin Elmer, Hitachi, Knauer, and Tosoh |
| *Vendors are placed in different tiers on the basis of their sales contribution to the overall revenues of the Indian HPLC systems market. | |||
| ADI Media Research | |||
After years of struggle, the Indian pharmaceutical industry finally got many things right in 2018 with the American government’s drugs regulator. Not only did inspection outcomes improve, far more in line with global outcomes, the number of cases where the Food and Drug Administration (FDA) there chose to classify a production unit here under the Official Action Indicated (OAI) category fell sharply.
From 27 cases of OAI in 2014 and 22 in 2017, the number shrank significantly to seven. After an inspection, the FDA classifies a plant as either OAI, VAI (Voluntary Action Needed) or NAI (No Action Needed).
This change came even as FDA-registered drug facilities rose 63 percent in India between 2011 and 2018. In comparison, there was a 51 percent increase in China, of 25 percent in the European Union and a 10 percent decline in the US itself.
 Technology trends
Technology trends
Recently, supercritical fluid chromatography (SFC), as an alternative technique to liquid chromatography (LC) and an extension of GC, has aroused extensive attention due to its high efficiency and environment protecting. Incorporating both the features of liquid and gas, SFC is regarded as a hybrid of GC and HPLC and exhibits a lot of advantages, such as high separation efficiency, low organic solvent consumption, and short separation time.
HPLC-MS based methods without derivatization are widely used in the analysis of lipids. The main advantage of HPLC over GC is the greater sensitivity as well as the enhanced chromatographic selectivity achieved with a rich variety of packed HPLC columns. However, HPLC for lipid analysis always takes a long time and is organic-solvent consuming. In recent years, SFC has met with great favor in targeted and untargeted lipid profiling due to its high efficiency, low organic-solvent consumption, and the specialty for unambiguous identification of the isomeric species of some lipids.
SFC is considered as a powerful tool for the analysis of the lipid profiling in biological samples. Owing to the advantages of a hybrid of GC and LC, SFC incorporates many features of these two techniques and shows outstanding separation efficiency. And the modifier can flexibly adjust the polarity of the mobile phase in SFC, providing the convenience for simultaneous analysis of various lipids with a wide range of polarities. In recent years, UHPSFC using columns with sub-2 µm particles shows a great potential as the comprehensive and high-throughput method for the analysis of lipid profiling. The development of new stationary phase is conducive to the separation of the specific analytes, especially for the natural isomers in biological samples. Recently, researchers have developed widely targeted quantitative lipidomic methodology using SFC-MS/MS, which represents a potentially useful tool for in-depth studies focused on complex lipid metabolism and biomarker discovery. Moreover, the online SFC technologies could greatly shorten the analysis time and simplify the pretreatment, so they show broad prospects in the lipid profiling. It is believed that the SFC-based method as a promising strategy could provide us many useful insights into lipid metabolism in physiological or pathological studies.
Way forward
To close, HPLC/UHPLC columns have been brought to the forefront of separations technology. Column reproducibility has improved, column stability/lifetime has increased, convenient connectivity has been mastered, efficiency has increased by orders of magnitude, a wide variety of regular and specialty stationary phases have brought difficult separations into the routine lab, and cost/injection has decreased.












