Defibrilators
ICDs – Gradually Gaining Share
The invention of ICDs was a milestone in cardiology, as it allows for the prevention of sudden arrhythmic death in many patients. However, unmet needs concerning ICDs remain.
Over the past decade, the global defibrillators industry has witnessed remarkable growth. And it is forecasted by Market Watch that the market will continue to grow at 5 percent per annum in the following decade. The major drivers for the market include the development of technologically advanced machines, rapid growth in the aging population with high risk of target diseases, rising incidences of cardiovascular diseases across the globe, growing focus of public and private organizations and key market players toward public access defibrillation, and increasing number of training and awareness programs across the globe.
Implantable cardioverter defibrillator (ICD) is much more popular than its counterpart, the external defibrillator. The possibility of remote patient monitoring by the utility of this device is a major driver of the segment. New-generation ICDs, with the bi-functional capability of preventing cardiac arrest along with performing the role of a pacemaker, are expected to enhance sales of the device. External defibrillators are preferred when there is a need to diagnose early onset of ventricular tachycardia and ventricular defibrillation.
The key players are focused on new product launches and strategic collaborations to strengthen their market positions. For instance, in May 2019, the US Food and Drug Administration (FDA) gave approval to Boston Scientific’s Emblem S-ICD that is used in the treatment of cardiovascular diseases. Medtronic is developing an extravascular (EV) ICD system, an innovative subcutaneous ICD that is designed to terminate arrhythmias, post-shock pacing to protect from sudden cardiac arrest, and temporary back-up bradycardia pacing.
The leading five companies, Medtronic, St. Jude, Medical, Boston Scientific, Biotronik, and Physio-Control command a combined share of 65 percent of the market. Currently, North America is the largest market of defibrillators worldwide, followed by Asia-Pacific.
Indian market dynamics
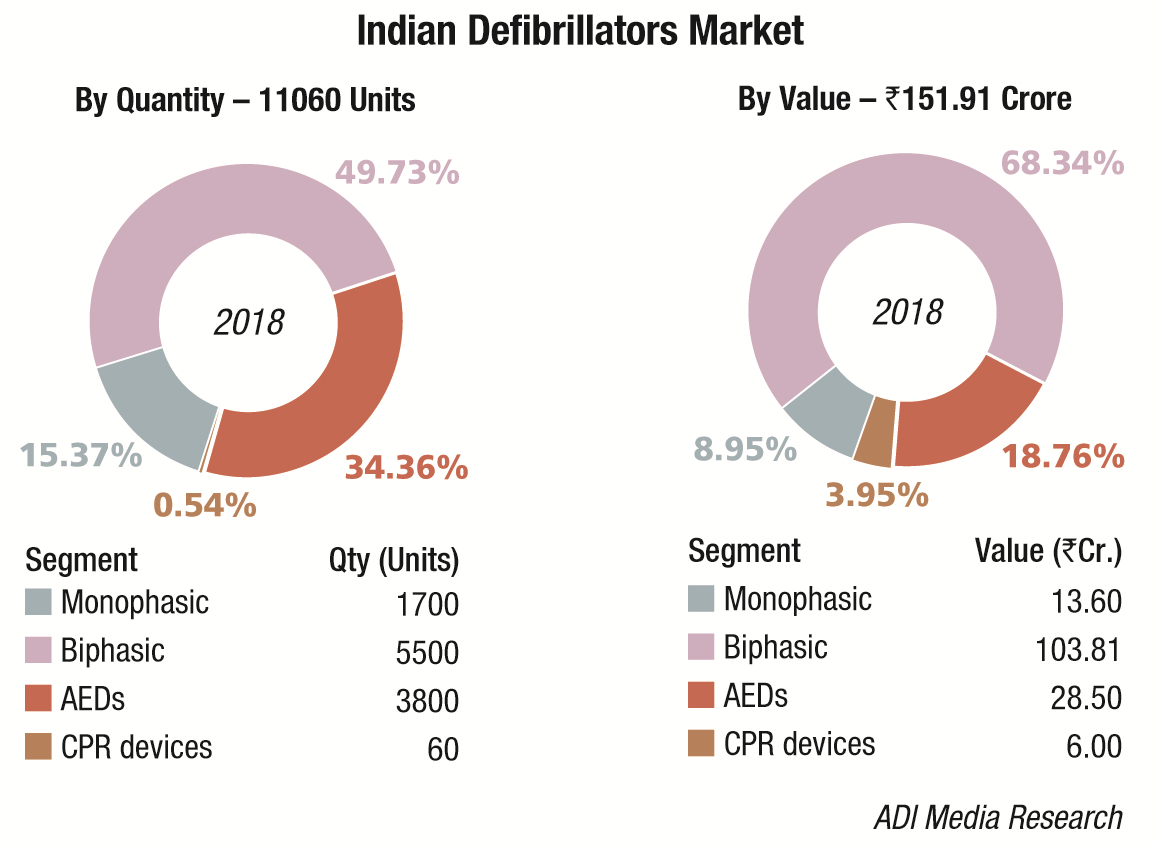
The Indian defibrillators market in 2018 is estimated at Rs 151.91 crore, and 11,060 units. Biphasic defibrillators, the highest priced in this segment, dominate the market with a 68 percent market share by value and 50 percent market share by volume. The government (HLL), state hospitals, and DGMS (Defense) are the main buyers. Seventy-five percent of the segment, priced at an average unit price of Rs 135,000, is the four-in-one design models, including monitoring, manual defibrillator, AED, and pacer with an effective IT solution, avoiding manual recording, improving efficiency, and reducing the workload of clinical staff. Accessories as ECG, SpO2, CO2, and NIBP may be added on to the system. Twenty-five percent of the segment is constituted by biphasic models priced at an average unit price of Rs 350,000, which also offer the CPR function.The monophasic defibrillators market continues to decline at about 10 percent every year.
Market – 2018* |
|||
|---|---|---|---|
| Tier I | Tier II | Tier III | Others |
| Mindray | Philips, Nihon Kohden, and Schiller |
Stryker (Physio-Control) | Zoll, BPL, Skanray, Mediana, and Beijing M&B; Lifepac AEDs (marketed by Medtronics) |
| *Vendors are placed in different tiers on the basis of their sales contribution to the overall revenues of the Indian defibrillators market. | |||
| ADI Media Research | |||
The AEDs market continues to grow by 20 percent in volume terms; in value terms the segment continues to hover in the vicinity of Rs 28 crore. With the airports covered now, the Indian Railways are planning to install AEDs at every railway station.
The year 2018 saw the acceptability of mechanical CPR devices. This segment is expected to pick up in 2019. In cardiac arrest, high-quality cardiopulmonary resuscitation (CPR) is a key determinant of patient survival. However, delivery of effective chest compressions is often inconsistent, subject to fatigue, and practically challenging. Mechanical CPR devices provide an automated way to deliver high-quality CPR. In situations where high-quality manual chest compressions cannot be safely delivered, the use of a mechanical device may be a reasonable clinical approach. Examples of such situations include ambulance transportation, primary percutaneous coronary intervention, as a bridge to extracorporeal CPR, and to facilitate uncontrolled organ donation after circulatory death.
 Technology Trends
Technology Trends
ICDs are routine practice for primary and secondary prevention of sudden death in selected patients with heart disease. Notwithstanding a reduction in the general cardiovascular disease mortality in recent decades, the global burden of sudden cardiac death (SCD) has not greatly diminished. The reason for this paradox is partially explained by the general aging of the population, as the elderly have a higher risk of SCD. Despite an 80-percent prevalence of severe coronary heart disease (CHD) underlying SCD, the majority of cases occur in patients with unknown heart disease, who do not have a current indication for an ICD.
ICD engineering has developed at a fast pace to counteract these limits (e.g., cardiac resynchronization therapy (CRT) to improve systolic function in severe heart failure, ICDs with bicameral pacing function to prevent the pacemaker syndrome, telemetry for the early recognition of arrhythmias and device malfunctions); however, further developments are required.
Remote monitoring. Device manufacturers have developed remote-monitoring systems for online transmission of data regarding the ICD functioning, including lead malfunctions (e.g., abrupt increase in impedances and pacing thresholds), battery end of life, and appropriate/inappropriate therapy. Real-time transmission of alerts on rhythm and technical parameters allows the clinician to make an early diagnosis and treat most problems of ICDs, with significant reduction in mortality. Another advantage of telemetry is the possibility of monitoring parameters connected with worsening of heart failure, such as heart rate and measures of thoracic congestion. Early identification of patients at increased risk for hospital admission for heart failure may prompt an ambulatory visit for reassessment of therapy. Given the complexity of heart disease and comorbidities in the majority of patients living with an ICD, it seems appropriate that future devices are capable of remote transmission of multi-parameter data, together with a risk score of clinically significant endpoints (e.g., hospitalization, arrhythmias, and death) to render a clinical reassessment more rapid when it is needed. A further example of how useful the daily remote transmission of ICD-collected data can be has been shown by Shakibfar and colleagues – machine learning algorithms based on data, such as percentage of ventricular pacing and daytime activity, were capable of predicting electrical storms, which are three or more episodes of sustained VT or VF occurring in 24 hours, which constitute a medical emergency.
Future cardiologists are required to become acquainted with online platforms for the follow-up of patients with ICDs; these are going to be implemented by all manufacturers. Paralleling the development of this network, engineers have to face the low but possible menace of cybersecurity breaches. Even though no cases have been reported up till now, it seems possible that hackers could interrupt the communication between the device and the remote server, with potentially dangerous clinical consequences. For this reason, St. Jude Medical has developed firmware upgrades to include protection against hacking.
Device infections. Microbial colonizations involving the leads pose a high risk for endocarditis and sepsis. The majority of these episodes are encountered in patients with comorbidities and who are immunocompromised. These conditions increase the risk for life-threatening infections, which occur in about 1.2 percent of implant patients, and require long-term antibiotic therapy. Device infections can occur even years after implantation, as a result of sepsis and colonization by microbial agents. In recent years, subcutaneous ICDs (S-ICDs), which do not require transvenous leads, have been associated with a better safety profile than conventional ICDs in terms of sepsis and endocarditis.
In future, randomized clinical trials, evaluating S-ICDs in patients at high risk of infection or with a previous conventional ICD explanted because of infection, will clarify the utility of this new generation of devices in this subset of patients. The use of an absorbable antibacterial envelope during implantation of conventional ICDs and pacemakers has shown promise in reducing the rate of electronic device infections.
However, the envelope does not provide protection for transvenous leads, which are the most dangerous site of microbial colonization, and costs, which are high, limit its use to patients at high risk. For these reasons, device infections remain an issue.
Subcutaneous ICD. The ICD lead, may often be damaged and constitute a source of infection. The metallic coil itself facilitates the formation of adhesions within the vasculature, which may render extraction more difficult when it is necessary. Nowadays, both mechanical and laser methods for lead extraction have been developed. According to the ELECTRa registry of transvenous lead extraction outcomes of the European Heart Rhythm Association (EHRA), complication rates (major complications requiring surgery or resulting in death) vary between 2 percent and 4 percent, depending on the center’s experience. However, there are few centers that have the appropriate training to perform lead removal, which is a challenging procedure in most instances. Transvenous leads also carry the risk of subclavian vein stenosis (26 percent of cases) or occlusion (9 percent),
which render more difficult not only the eventual lead extraction but also reimplantation.
In a clinical scenario, in which leads have been removed, it remains possible to implant a subcutaneous ICD (S-ICD). The EMBLEM MRI S-ICD system is the latest device produced by Boston Scientific, which today is the sole manufacturer commercializing S-ICDs approved for patients. The device is MR-conditional and it is entirely subcutaneous, with a subcutaneous lead positioned vertically in the precordial position. Apart from lacking a transvenous system, which renders it more suitable for patients with a history of infection and for the young, being a less invasive device, it is larger than the conventional ICD generator to ensure transthoracic shocks of 80 J (conventional ICDs produce shocks of about 35 J) and the duration of battery is usually shorter (about 7 years). Technical characteristics have improved from the first-generation models. Remote transmission of data is also possible.
The principal limit of S-ICDs is the lack of permanent pacing function, a characteristic that is present in conventional ICDs. For this reason, the choice of implanting an S-ICD should be carefully balanced against the risk of developing a bradyarrhythmia requiring pacing. Younger patients have a lower likelihood of developing the need for permanent pacing and are usually the best candidates for S-ICD implantation.
Eligibility assessment with basal and exercise stress ECG pre-implant is recommended to avoid potential T-wave oversensing and consequent inappropriate therapy, which is a problem in a relatively high percentage of patients, especially in those with Brugada syndrome and prominent T-wave alterations. The manufacturer has developed a new algorithm (SMART Pass) that seems effective in reducing oversensing, but further real-world experience with these devices is required.
Wireless CRT. The most recent innovation, still in phase II clinical research, is wireless cardiac resynchronization therapy, which could be a future option for patients who are candidates for CRT-P (pacemaker) or CRT-D (defibrillator). In detail, the SELECT-LV study evaluated the feasibility of a new-generation biventricular stimulation in a few patients (n=35) with heart failure and failed conventional CRT. The left ventricular stimulation WiCS-LV system (EBR Systems Inc., Sunnyvale, CA, USA) converts ultrasound impulses produced by a subcutaneous generator into electrical stimulation, synchronized to right ventricular pacing via a mini-electrode implanted in the endocardium of the left ventricle.
This system has also been successfully applied to a patient having a right-ventricular leadless pacemaker, to produce a completely leadless biventricular pacemaker. Other systems are going to be developed, for example, using three leadless pacemakers (for right and left ventricles and right atrium) communicating via low-voltage alternating currents (conductive intracardiac communication).
Way forward
The invention of ICDs was a milestone in cardiology as it allowed for the prevention of sudden arrhythmic death in many patients. However, unmet needs concerning ICDs remain. The clinical cardiologist still has to face inappropriate shocks, lead failures, device infections, and electrical storms. Many of these issues can be solved or improved with the use of current technology, but nowadays ICDs are far from perfect. The knowledge of ICD management and their limits is also the basis for future engineering and the development of new-generation devices such as the S-ICD and wireless CRT.












