Life Sciences
Is automation the key to LC-MS?

As clinical laboratories embrace semi-automated solutions for mass spectrometry-based assays, a key question remains: Could a fully automated mass spectrometry analyzer boost adoption of this technology?
Thompson’s first description of a mass spectrometer in 1913 was eventually followed by the introduction of time-of-flight (TOF) instrumentation in the mid-60s; however, 20 more years passed before a TOF instrument would become commercially available with broader acceptance. Magnetic sector instruments provided sufficient resolution for accurate mass measurements but were lacking sensitivity in scanning mode, as the resolution is in reverse proportion to the sensitivity. Fourier-transform ion-cyclotron resonance (FT-ICR) instruments with high resolution as well as sensitivity were an alternative to the magnetic sector instruments, but the new TOF instruments were also an appropriate answer, showing a resolving power (m/Δm) approaching that of the magnetic sector instruments. However, with further improvements, including the reflector (or reflectron) and the now commonly adopted orthogonal acceleration (OA) design, TOF instrumentation has effectively replaced the once dominant magnetic sector instrumentation since the millennium.
The first component of the mass spectrometer is the ion source, where analytes are ionized. The most typical ion source used is electrospray ionization (ESI), but other techniques, such as atmospheric pressure chemical ionization (APCI) or atmospheric pressure photo ionization (APPI), are available. After the ionization process, ions are guided through a capillary into the vacuum chamber. The next elements encountered in the ion optics are the quadrupole, the collision cell, the pulser, a reflector (reflectron) in the flight tube, and the detector. The separation of ions in the flight tube based on different arrival times at the detector is the fundamental principle of TOF instruments. The signal from the detector is then processed to show a final m/z spectrum. These elements are common to all TOF instruments but vary in the details and modifications offered by different vendors. Other HRAM spectrometry instruments include FT-ICR and the electrostatic ion trap, with the latter taking a large portion of the FT-ICR market since its introduction.
In the last 20 years, mass spectrometry has evolved from purely academic instrumentation to a technique now present in most analytical laboratories. Together with ongoing improvements in seamless software workflows and capabilities, increases in sensitivity and resolution are key drivers for this development.
Indian market
The Indian mass spectrometers market is estimated at ₹472 crore in 2020 with 299 units. In 2020, the single and triple quadrupoles saw traction in 2020 over the other segments. While globally the triple quadrupoles are increasingly preferred over their single counterparts, India being a price sensitive market finds adequate application for both the products. On the same logic, globally the trend is moving toward HR-MS. It allows detection of analytes to the nearest 0.001 atomic mass units and measures the exact mass of analytes without fragmentation, and can be combined with a quadrupole in which case fragmentation is also possible and can add more selectivity to the method. However, in India, the clinicians are looking to only perform targeted quantitation and they are procured largely to meet regulatory compliance.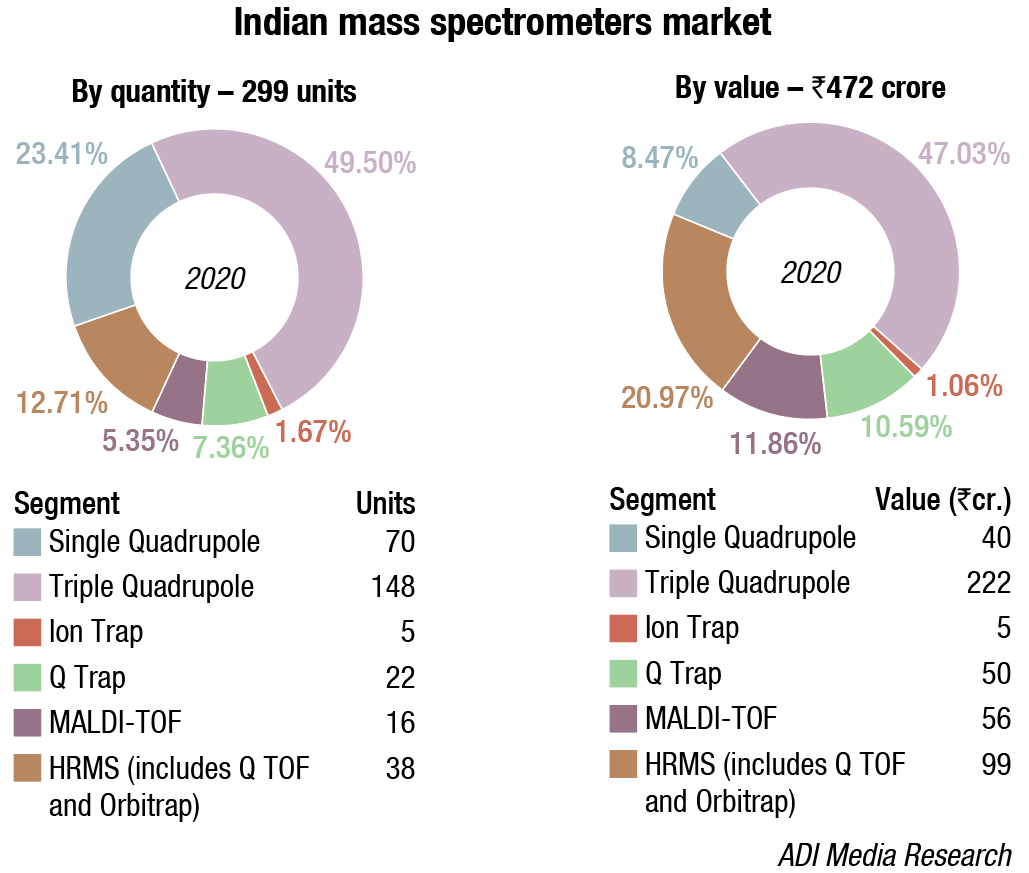
Researchers at Institute of Genomics and Integrative Biology (IGIB) and the National Centre for Disease Control (NCDC) were able to detect novel coronavirus with 95 percent sensitivity and 100 percent specificity with respect to RT-PCR. Detection of the virus takes less than three minutes; time from sample preparation to detection takes less than 30 minutes.
The new method can directly detect the virus without amplifying the RNA for detection, as is the case with RT-PCR. The new method relies on detecting the presence of two peptides which are unique to SARS-CoV-2 virus and not seen in any other coronavirus or other viruses. Though seven peptides were found to be unique to SARS-CoV-2, only two peptides are used for quick virus detection. One of the peptides is the spike protein and the other is a replicase protein. The unique peptides were seen in over 54,000 genomic sequences of the SARS-CoV-2 virus deposited in a public database (GISAID) as on July 1.
“The sensitivity is 90.4 percent when compared to RT-PCR if we have very high stringency. By that we mean if the ratio of signal to noise is 10-fold. If we use the standard 3.3 times signal to noise ratio (where signal peak is 3.3 times higher than the noise) then the sensitivity is 95 percent,” says Dr Shantanu Sengupta from IGIB, who led the team.
| Segment | Major Players |
|---|---|
| Single Quadrupole | Waters, Shimadzu, Agilent, Advion, and Thermo Fisher |
| Triple Quadrupole | SCIEX, Waters, Shimadzu, and Agilent |
| Ion Trap | Thermo Fisher and Bruker |
| Q Trap | SCIEX |
| MALDI -TOF & Maldi Top TOF |
Bruker, Shimadzu, and JEOL |
| HRMS (includes Q TOF and Orbitrap) |
SCIEX, Waters, Agilent, and Thermo Fisher, Bruker, and Shimadzu |
| ADI Media Research | |
The results have been published in the Journal of Proteins and Proteomics. “We could detect the peptides of SARS-CoV-2 virus even in patients who have recovered from the symptoms and have tested negative for the virus by RT-PCR. The peptides were present even after 14 days of initial infection. This highlights the sensitivity of the technique.”
The virus in the swab samples are inactivated using detergent and further processed before being used for virus detection.
The researchers initially tested the technique using 14 nasopharyngeal swab samples and then confirmed using 20 controls and 63 samples from COVID-19 patients who tested positive by RT-PCR and subsequently recovered.
“The mass spectrometer is expensive but it would cost only about `100 per test, and so cheaper than RT-PCR. Many research labs have the mass spectrometer,” says Dr Sengupta. “Since it takes less than 30 minutes to detect the virus and is also highly sensitive and specific, it can be used for screening and diagnostic purposes. It can either complement RT-PCR or be used as an alternative to RT-PCR.”
Government initiatives for pollution control and environmental testing are to some extent supporting the growth of this market.
However, the dearth of skilled professionals and the high cost of instruments may restrain the market to a certain extent.
Global market
The mass spectrometry market size is expected to grow from an estimated USD 4.1 billion in 2020 to USD 5.6 billion by 2025, at a CAGR of 6.5 percent. Increasing spending on pharmaceutical R&D across the globe, government regulations on drug safety, growing focus on the quality of food products, increase in crude and shale gas production, and growing government initiatives for pollution control and environmental testing are high growth prospects for the mass spectrometry market during 2020–2025.
The unexpected outbreak of COVID-19 has significantly affected the mass spectrometry market. The market is expected to witness a diverse set of adoption in 2020. Mass spectroscopy adoption for testing applications is high in healthcare and pharmaceuticals, biological research, and food and beverages industries. New drug development, and drug repurposing, and the increasing production of pharma formulations have led to an increasing need for safety and quality measures in the pharma industry. Stringent government regulations and increased demand for quality maintenance in these industries drive the adoption of mass spectrometry. However, supply chain disruptions in the petrochemical industry, and regulatory relaxation in pollution monitoring is expected to limit the adoption of mass spectrometers.
Ladder to next generation clinical testing
Bhaumik Trivedi
Sr Application Scientist
Shimadzu Analytical (India) Pvt. Ltd.
The advancement of mass spectrometry (MS) technology along with the development of new applications has accelerated the incorporation of MS into more areas of IVD (In-vitro diagnostics). Hence clinical laboratories have been exploring various types of MS as powerful analytical tools for confirmation purposes.
The invention of ElectroSpray Ionization (ESI) and Matrix-assisted Laser Desorption/ionization (MALDI) techniques were undoubtedly the milestones in the history of mass spectrometry (MS), fully deserving the Nobel Prize awarded in year 2002. ESI enabled high-efficiency ionization directly from a liquid phase, which made liquid chromatography mass spectrometry (LC-MS) the most sensitive and quantitative method for a wide variety of applications. On the other hand, MALDI enabled direct ionization of biomolecules and significantly reduced the sample preparation burden for analyses requiring high throughput. Whilst advancements in mass spectroscopic techniques has continuously improved the quality of data, it was the new ionization method that drastically changed the landscape of MS applications.
In this regard, these latest technologies have the potential to pioneer new types of applications with the new ionization interface. The technique successfully incorporates the best of both worlds; high ionization efficiency of ESI and simplicity of MALDI. This was achieved by using an ultrafine needle to take an extremely small volume of sample on its surface and subsequently applying high voltage to the needle to imitate an ESI probe. The applied voltage allows electron transfer from the solvent and causes migration of solute as well as solvent towards the needle-tip by electrostatic repulsion, which eventually form a jet of nano-droplets and a Taylor cone like observed in a normal ESI process. The new ionization technique was named Probe-ESI (PESI).
PESI technology implemented, is characterized by the microscopic sampling volume and high sensitivity for the amount sampled. In a typical application, compounds at a low ng/mL concentration range can be readily detected, in which case the actual abundance of compound subjected to MS can be as small as a few attograms. For this, such technique can be a robust solution for the routine analysis of high-complexity matrices like serum, plasma, as contamination of MS hardware can be perfectly mitigated. Moreover, direct sampling from solid samples causing minimal destruction might enable new applications such as real-time analysis of live tissues or cells as well.
The R&D expenditure of pharmaceutical companies has increased significantly over the last two decades. Research activities in the pharmaceutical and biotechnology industries are driven by investments in key areas, such as biopharmaceuticals and personalized medicine. According to the EU Industrial R&D Investment Scoreboard, the pharmaceutical and biotechnology sector amounts to 18.9 percent of total global R&D expenditure. Mass spectrometry plays a key role in the pharmaceutical industry, from the early stages of drug discovery to late-stage development and clinical trials. Thus, increasing funding in the pharmaceutical and biotechnology industry is expected to drive the growth of the mass spectrometry market.
Spectrometry instruments are equipped with advanced features and functionalities and are thus priced at a premium. Apart from the system’s cost, the cost of compliance of the system to industry standards is also very high. Pharmaceutical companies require many such systems, and hence, the capital cost increases significantly. Furthermore, academic research laboratories find it difficult to afford such systems, as they have controlled budgets. These are the major factors limiting the adoption of mass spectrometry systems among end users.
China and India present various opportunities for the growth of the mass spectrometry market, and together, generate a huge demand for single mass spectrometers and hybrid spectrometry instruments due to the greenfield projects being set up in various end-user industries in these countries. The biopharmaceutical industry in these countries is robust and is expected to contribute largely to the growth of the spectrometry and chromatography markets. Key industry players are establishing new facilities, R&D centers, and innovation centers to capitalize on this opportunity and engaging in collaborations with players in the Asian market.
Skilled personnel with relevant experience and knowledge are required for the efficient use of spectrometry equipment. Errors such as misplacing a sample in GC-MS or LC-MS or issues such as fingerprints or bubbles in the solution can impact the quality of the final result. Moreover, in mass spectrometry, sample preparation (including aliquoting, dilution, and extraction) is a key step in isolating the analyte of interest. It eliminates interferences that could affect the precision of the result. The lack of knowledge regarding the right choice of technology also affects results and may incur direct and indirect expenses for end users. There is currently a dearth of skilled personnel for method development, validation, operation, and troubleshooting activities, which is expected to restrain the growth of the mass spectrometry market.
The hybrid mass spectrometry segment is expected to witness the fastest growth in coming years. Advantages offered by hybrid mass spectrometers, such as rapid and high-resolution testing abilities with more accurate and precise results. Consequently, the demand for mass spectrometry devices for high throughput screening is also growing. The hybrid mass spectrometry segment is further divided into Triple Quadrupole, Quadrupole ToF (Q-ToF), and Fourier Transform Mass Spectrometry (FTMS).
By application, the mass spectrometry market has been segmented into life science research, drug discovery, environmental testing, food testing, applied industries, clinical diagnostics, and other applications. Among these, the life science research segment dominated the market in 2020. The increasing application of omics technology in diagnostics and biomarker identification and the increasing R&D expenditure and government funding for proteomics are expected to drive the market for this segment.
North America is estimated to be the largest market. It is driven primarily by the growing funding for research and government initiatives in the US, widespread usage of mass spectrometry in the metabolomics and petroleum sector, and CFI funding toward mass spectrometry projects in Canada. In addition, regulatory agencies in the US, such as the FDA, are encouraging the use of analytical techniques to ensure that the pharmaceutical products released in the market adhere to quality requirements. Lately, the US has seen a significant increase in the shale gas and crude oil production with increasing oil fields and this has resulted in subsequent increase in the employment of the mass spectrometer.
Dr Aarti Khanna Nagpal
Lab Head and DGM
SRL Diagnostics
Mass spectrometers operate by converting the analyte molecules to a charged state, with subsequent analysis of the ions on the basis of their mass to charge ratio. MS, when coupled to gas chromatography or tandem MS, coupled with liquid chromatography, provides very specific analysis of analytes. MS can outperform traditional biochemical measurements used in diagnostics with its ability to detect trace level of compounds. This technology is being used for a wide range of applications such as NBS using dried blood spot, therapeutic drug monitoring, endocrinology, MALDI-TOF (time-of-flight) for clinical microbiology, vitamin D, alcohol and steroid assays to name a few. Although MS continues to make significant contributions to diagnostics, there are substantial challenges including cost of equipment, requirements for skilled staff and regulatory uncertainty that need to be understood before its implementation. Despite these challenges, clinical applications of MS continue to expand and in the coming future it will be used in almost all areas of laboratory medicine.
The major players in the mass spectrometry market are Thermo Fisher Scientific, Sciex, Agilent Technologies, Waters Corporation, PerkinElmer, Shimadzu Corporation, Bruker, Analytik Jena, JEOL, Rigaku, Dani Instruments, Leco, and Hiden Analytical.
The road to automation in clinical mass spectrometry
As clinical laboratories embrace semi-automated solutions for mass spectrometry-based assays, a key question remains: Could a fully automated mass spectrometry analyzer boost adoption of this technology?
Drug testing and determining 25-hydroxyvitamin D (25(OH)D) levels are the two main uses for liquid chromatography-tandem mass spectrometry (LC-MS/MS) at large commercial laboratories, health systems, and drug testing laboratories. Some laboratories also deploy LC-MS/MS for neonatal metabolism screening, endocrine testing, and detecting monoclonal immunoglobulins.
Current LC-MS/MS methods are often lab-developed tests (LDTs) whose development and implementation can be challenging for some clinical laboratories because of labor-intensive and mostly manual workflows. To establish accurate and reproducible MS-based LDTs, a laboratory requires a significant level of expertise. Consequently, LC-MS/MS has not gained traction in hospital laboratories as much as had been anticipated a decade ago.
While the clinical use of LC-MS/MS technology is growing, most laboratories still rely on immunoassay-based methods for the majority of their workload. Ten or 15 years ago, many people thought LC-MS would offer a revolution in hospital laboratory testing and that LC-MS would rapidly replace immunoassays, but that has not happened.
Clinical laboratorians prize both the sensitivity and versatility of LC-MS/MS. Development of automated solutions in LC-MS/MS primarily has been focused on pre- and post-analytical areas. In recent years, some integrated systems to automate preanalytical steps in LC-MS/MS have been introduced by Shimadzu, Thermo Fisher, Tecan Group, Agilent, SCIEX, Waters, and Aurora.
Until recently, no fully automated LC-MS/MS system was available in the United States, but that changed in March with Thermo Fisher’s announcement with the Cascadion SM Clinical Analyzer, a fully automated system. Thermo Fisher first introduced this analyzer in Europe. Due to the highly competitive nature of innovation in this part of the industry, it is not easy to forecast which company will be the next to introduce such an instrument.
Currently, the only test that can be run on Thermo Fisher’s analyzer is the Cascadion SM 25-Hydroxy Vitamin D Assay. The company is developing an immunosuppressants panel for the analyzer.
Roche is also working on an LC-MS/MS automation module for its Cobas pro analyzer.
Is this a game changer?
Might the introduction of a fully automated LC-MS/MS analyzer be a game changer for clinical diagnostics?
Clearly, a fully automated LC-MS/MS solution has the potential to change clinical diagnostics. LC-MS/MS offers sensitivity and specificity not achievable with current automated chemistry platforms. Currently, most LC-MS/MS assays are run in a batch mode, and consequently turnaround times suffer. The ability to offer LC-MS/MS in a random-access high-volume analyzer will improve both turnaround times, as well as improve sensitivity and specificity of the assays. The full impact of an automated LC-MS/MS system will depend on how feasible it is to install in a routine clinical laboratory. The hurdles to overcome include instrument challenges, assay menu, price, and ease of use.
A major driver for hospital labs to adopt Cascadion will be availability of a test menu with which immunoassays cannot compete. This could be through consolidating multiple tests on a single platform, significant differences in selectivity, or by saving money from internalizing testing previously sent out to reference laboratories.
Dr Jyoti Upadhyay
Assistant Professor, School of Health Sciences
University of Petroleum and Energy Studies
Mass spectrometers play an important role from the early stages of drug discovery to late stage developments and clinical trials. Advanced features and functionalities offers precise and high resolution test results encourage customers to opt for this equipment. The growing demand for tandem and hyphenated techniques especially from pharmaceutical, biotechnology, food and beverages is driving the market. The hybrid mass spectrometry segment including triple quadrupole, quadrupole ToF (Q-ToF) and fourier transform mass spectrometry (FTMS) is expected to witness highest compound annual growth rate (CAGR) from 2020 to 2025. Increasing demand of mass spectrometry in omics technology in diagnostics and biomarkers like proteomics, metabolomics are expected to drive the market for this segment.
References laboratories have large 25(OH)D testing volumes so might be able to justify the analyzer for just that one assay. But an instrument with only one FDA-approved test initially might be a tougher sell to smaller laboratories.
Once there are two or three high-workload tests available on analytical platforms, it is easier to financially justify adding more expensive, low-volume tests on that same instrument, perhaps assays that your health system wants but has not previously been able to afford.
The main benefits of having a fully automated LC-MS/MS platform would be the ability to bring send out testing back in-house and improve turnaround times.
While only being able to run one test might limit current marketability, availability of a fully automated LC-MS/MS solution is a start in making this technology more widely available to clinical laboratories. This represents a step in the right direction. As more tests are made available on fully automated systems, LC-MS/MS technology will move closer toward more widespread adoption.
Advances in clinical mass spectrometry
Clinical applications of mass spectrometry continue to expand. Arguably, one of the most exciting areas where clinical mass spectrometry is making inroads is precision medicine and the transition from sick care to well care, through tracking of biomarkers that objectively measure wellness and the early onset of diseases.
The power of this technique lies in its speed, high throughput, specificity, and ability to identify, quantitate, and characterize unknown molecules.
Historically, MS was limited to small molecules. However, thanks to recent expansions of the technique, a myriad of biological samples can now be studied. The invention of matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS is one such advancement. While traditional ionization methods use harsh thermal vaporization techniques, making them incompatible with the characterization of large biomolecules, MALDI-TOF resolves this issue by embedding the sample in a small organic compound matrix before using an ultraviolet laser to ionize and desorb the sample. Another is the addition of a liquid chromatography (LC) step to physically separate the sample before subjecting it to electrospray ionization and analysis by MS. This technique often also incorporates a tryptic digest step (LC-MS/MS).
These advancements in MS technology have provoked an upsurge in the potential applications of MS in biological laboratories–from identification of microbes, to shotgun proteomics studies, to personalized medicine and many more. Mass spectrometers are fast becoming an essential enabling technology for the next generation of modern medicines and an integral component of clinical laboratories, owing to their versatility, specificity, high throughput, and ability to detect and quantify unknown components and highly complex samples.
Point-of-care diagnostics. The versatility and accuracy of MS make it an interesting technique for point-of-care testing applications, where diagnostic tests are administered at the time and place of patient care, facilitating fast decisions and simplified healthcare delivery. However, in their full-sized form, certain practical aspects limit the feasibility of this application, including the cost, size, and complexity of the instrument. For these reasons, simplified, miniaturized versions of mass spectrometers have captured the interest of many scientists. Indeed, benchtop mass spectrometer prototypes have been built and tested. Such devices are intended for the biologists’ bench, the physician’s office or even inside ambulances, ultimately allowing the conversion of blood, urine or tissue samples into clinical diagnoses in situ. To date, these machines remain in the prototype stage of development, but could represent the last piece of the puzzle to realize the full potential of mass spectrometry in the clinic.
Rapid identification of microbes. Screening for microbial infection is an imperative requirement in the clinical laboratory. While the gold standard has long been cell culture, this method suffers from long wait times for the results and poor sensitivity, which can result in false negatives. For example, blood cultures may only show a positive result in 10–15 percent of patients with severe pneumonia. MALDI-TOF MS represents a viable alternative, which can analyze bacterial colonies and facilitate identification at the genera or species level within minutes, rather than days. More recently, MALDI-TOF techniques have been expanded to detect antimicrobial resistance in bacterial samples, for example, vancomycin-resistant enterococci and methicillin resistant Staphylococcus aureus. In response to the COVID-19 pandemic, novel methodologies to detect biological signatures associated with the coronavirus by MALDI-TOF are in development.
Monitoring of therapeutic antibodies. Monoclonal antibodies (mAbs) are a relatively new class of therapeutics used to treat a range of diseases, including autoimmune disorders and cancers. With the widespread use of antibody therapeutics in the clinic come new challenges for accurate monitoring. One such challenge is the difficulty in differentiating between the antibody drug and endogenous monoclonal proteins in the patient, such as interleukin-6. This can cause interference with biomarker panels used to monitor the patient, reducing their accuracy. This creates a need for new assays to monitor these biological drugs in the patient. Currently, enzyme-linked immunosorbent assays (ELISAs) are the most common method used by clinical laboratories to monitor mAbs in patients. However, their labor-intensiveness and propensity for false results are serious drawbacks, whereas, LC-MS and LC-MS/MS represent a highly sensitive and precise alternative, with high-throughput and very small sample volumes. While MS-based assays for monitoring therapeutic antibodies are not yet widely used, experts predict that MS will be the technique of choice as antibody technologies continue to evolve.
Proteomics of health and diseased tissues. Proteomics is the study of the entire complement of proteins in an organism, tissue or cell and their changes under different conditions. The functioning of tissues is ultimately dependent on the expression, regulation and alteration of its proteins, therefore proteomics data create a highly valuable window into the health of the tissue. Using this data, it has been possible to identify new biomarkers to non-invasively diagnose diseases, for example, C – reactive protein, and troponin I as biomarkers for myocardial infarction. However, the sheer complexity of biological samples and the low concentrations of many biomarkers therein test the limits of most analytical technologies.
Recent developments of MS technologies, such as automated parallel sample processing and multidimensional separation steps, have increased the throughput and sensitivity, making it an increasingly attractive candidate for proteomic studies. Furthermore, almost all biological samples can be studied by MS, meaning that simultaneous characterization of biomolecules at every omics level, from genome to lipidome, is feasible. In this respect, MS is one of the only techniques that can provide systems level data regarding the inner workings of tissues in health and disease.
Personalized oncology. Omics data provides unprecedented insights into disease, so it is unsurprising that there is a growing interest in leveraging this data to improve medical outcomes. Personalized medicine is a novel paradigm which centers on tailoring medical treatments to the individual characteristics of each patient, most often determined by genomic profiling. Early work in this field has primarily been focused in oncology, where patients who have cancers caused by specific genomic abnormalities are paired with targeted therapies that attack the molecular mechanisms behind their malignancy. More recently, however, the importance of proteomic and transcriptomic data to complement genomic information has been realized. For example, within a group of breast cancer patients whose tumors are HER2-positive (HER2+), there may be a range of HER2 protein expression profiles, which respond differentially to anti-HER2 therapy. The use of MS to quantitate HER2 protein expression levels is in development for clinical use and will allow clinicians to target anti-HER2 therapies based not only on the genetic signature but also based on the specific protein expression levels.
Way forward
The automation and integration that will transform LC-MS/MS into an integral and cost-effective part of medical diagnostics are technically feasible, as demonstrated by the partial automation on the market today. All that remains is for technology providers and regulators to step up and meet the demand.












