COVID-19
Mad rush anticipated for syringes
As the entire world anxiously awaits the breakthrough for the coronavirus vaccine, the disposable syringes manufacturers, hoping to capitalize on this, are planning on increasing production capacity levels.
The market for syringes worldwide, estimated at USD 16.1 billion in 2020, is projected to reach USD 25.1 billion by 2027, growing at a CAGR of 6.5 percent during 2020–2027. Of these, prefilled syringes are projected to grow at a 7.9 percent CAGR to reach USD 11 billion by 2027. After an early analysis of the business implications of the pandemic and its induced economic crisis from the coronavirus pandemic, growth in the disposable safety syringes segment is readjusted to a revised 5.7 percent CAGR for the next seven-year period.
The US market in 2020 is estimated at USD 4.35 billion, accounting for 27.04 percent of the global syringe market.
China
China is forecast to reach an estimated market size of USD 5.5 billion in the year 2027, trailing a CAGR of 10.2 percent through 2027. Canada is expected to grow at 5.9 percent, Japan at 3.4 percent, and Germany, which leads the pack in Europe, at 4.2 percent CAGR in the period 2020–2027. China and Rest of Europe are expected to be each at USD 5.5 billion in 2027.
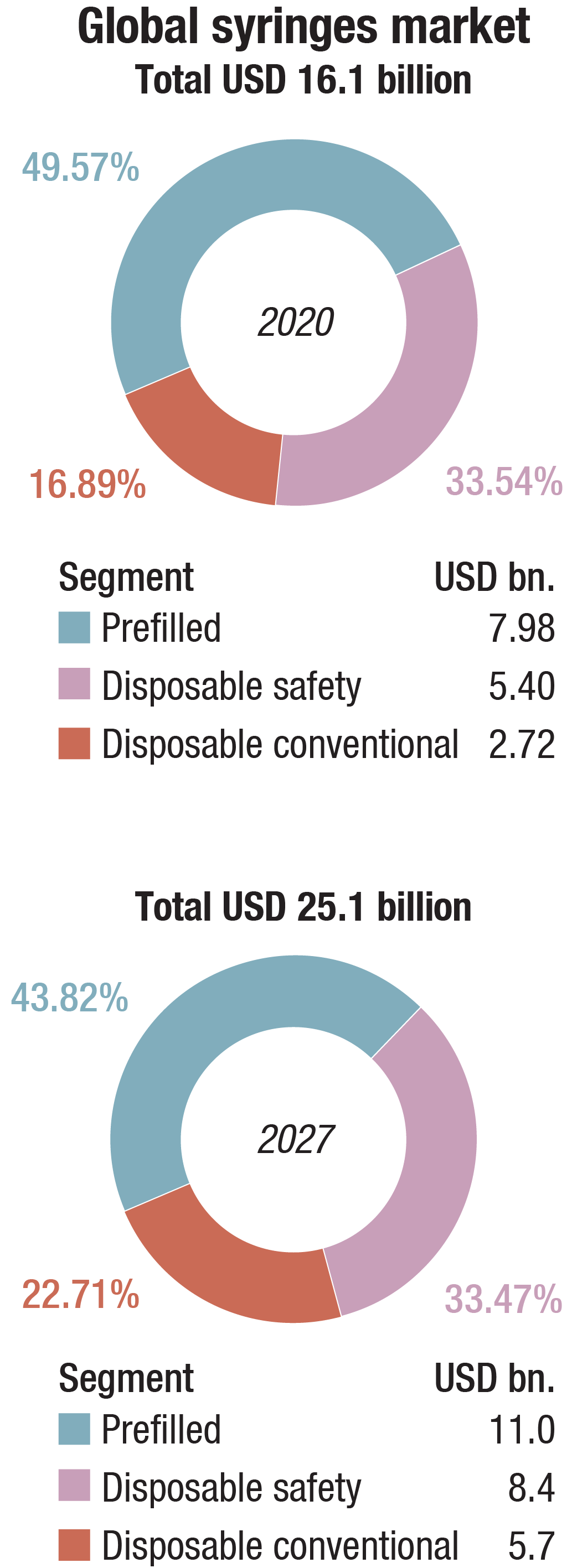 Disposable conventional syringes segment corners a 16.9 percent share in 2020. In the global disposable conventional syringes segment, the US, Canada, Japan, China, and Europe will drive the 6 percent CAGR estimated for this segment. These regional markets, accounting for a combined market size of USD 2.1 billion in the year 2020, will reach a projected size of USD 3.1 billion by 2027. China will remain among the fastest growing in this cluster of regional markets. Led by countries like Australia, India, and South Korea, the market in Asia-Pacific is forecast to reach USD 3.4 billion by the year 2027, while Latin America will expand at a 7.8-percent CAGR.
Disposable conventional syringes segment corners a 16.9 percent share in 2020. In the global disposable conventional syringes segment, the US, Canada, Japan, China, and Europe will drive the 6 percent CAGR estimated for this segment. These regional markets, accounting for a combined market size of USD 2.1 billion in the year 2020, will reach a projected size of USD 3.1 billion by 2027. China will remain among the fastest growing in this cluster of regional markets. Led by countries like Australia, India, and South Korea, the market in Asia-Pacific is forecast to reach USD 3.4 billion by the year 2027, while Latin America will expand at a 7.8-percent CAGR.
Major global players in this segment include Abbott Laboratories, B. Braun Melsungen AG, Becton, Dickinson and Company, Cardinal Health, Gerresheomer AG, Kawamoto Corporation, Medline Industries Inc., Merit Medical Systems, Nipro Medical Corporation, Retractable Technologies, Schott AG, Smiths Medical, and Terumo Corporation.
The smart syringes segment is anticipated to witness the fastest growth with the rising technological advancements and increasing incidence of needle stick injuries. WHO has recommended the usage of smart syringes to reduce contamination and transfer of harmful diseases, and is even urging for the exclusive usage of smart syringes.
Groundbreaking innovations, such as the introduction of two-step disposable auto-injector devices, are bolstering the growth of the global syringes market. Companies in the market for syringes are increasing efforts to introduce devices that cater to different viscosities in drugs. They are increasing their focus to deliver design improvements in devices that are eliminating the need for customizing system components. Novel two-step disposable auto-injector devices are overcoming challenges of breakage and incompatibility associated with poorly integrated systems.
With high prevalence of needlestick injuries worldwide, several millions of disposable syringes end up in trash dumps and landfills, resulting in increased environmental waste. Hence, companies in the disposable syringes market are innovating in needle-free injectors in order to eliminate environmental risks and improve patient experience. Needle-free syringes are reducing needle reuse activities in healthcare settings and playing a pivotal role in controlling the incidences of cross contamination. Needlestick injuries not only infect patients, but also expose healthcare workers to different types of blood-borne pathogens. As such, needle-free syringes are being extensively used to deliver vaccines to the proper tissue depth of patients.
The concept of specialty syringe production is growing popular in the market landscape. However, design and manufacturing challenges in specialty syringes like the product’s complex mechanism, as compared to standard syringes, are posing a hurdle for manufacturers.
Manufacturers are looking at utilizing the computer-aided design (CAD) geometry to overcome molding constraints in specialty syringes. They are conducting design reviews that involve risk evaluation of designs for specialty syringes.
COVID-19–The US case study
The US authorities are getting ready to distribute a COVID-19 vaccine as soon as it is developed, albeit they acknowledge that a mass vaccination campaign is going to be complicated. An estimated 700 million injections may be needed to inoculate the nation, at least two shots for every person, says a military document.
The US government is apparently buying a range of devices to deliver the vaccine because they are not sure what they need. And, the Trump administration is looking to boost domestic manufacturing.
The US government has invested USD 42 million to enable Becton, Dickinson and Company (BD) ramp up its production operations of syringes and needles in Nebraska, ahead of the future coronavirus vaccination push. The Defense Production Act designation for the order will allow the company to seek priority access to raw materials for manufacturing.
The increased capacity for manufacturing is expected to be functioning within 12 months, and will provide priority access to the US government for hundreds of millions of syringes and needles for COVID-19 vaccination efforts. A confirmed order has also been placed by the US government for 50 million needles and syringes, at a value of USD 11.7 million, to be delivered by the end of December 2020.
The government has also promised ApiJect USD 138 million to produce 100 million of its injectors by the end of the year, which will require the company to retrofit new manufacturing lines in the existing factories. And it has offered another USD 456 million as part of a public-private partnership contract to bring online several new factories to make another 500 million devices.
These amounts are more than double the per-syringe cost the government is paying other companies for the work.
ApiJect’s devices, still awaiting FDA approval, are self-contained, with soft plastic blisters that are squeezed, like a nose spray or eye drop, to push the vaccine through an attached needle and into the patient.
The manufacturer has started discussions with the USFDA to review the device on a priority basis while the company moves ahead fitting factories to make their injectors.
The device includes a little computer chip–like the ones in credit cards–that can transmit information about the drug, dose, location, and time of administration.
In early May, the government had put in two orders to Retractable Technologies in Little Elm, Texas, and Marathon Medical in Aurora, Colorado, totaling 320 million needles and syringes. And earlier this month, in July, Retractable entered into a second contract with the government, this one for USD 53 million meant to boost domestic manufacturing.
Together that sounds like enough injection devices!
But Retractable, which was worried enough about its financial future that earlier this year it received a USD 1.36 million loan from the Paycheck Protection Program, has been doing about 80 percent of its manufacturing in China. And Marathon is a medical supply distributor, and there is no indication on its web site that it manufactures needles and syringes at all.
Despite the race to replenish the domestic needle and syringe supply, about 400 shipping containers of syringes have left the US for countries, including Germany, Colombia, Australia, Brazil, and Italy this year, according to Panjiva Inc., a service that independently tracks global trade. That is the same, on average, as syringe exports over the past five years!
Second Opinion
Acquiring a quality lab test result
 Dr Tejal Bhatt
Dr Tejal Bhatt
Consultant Pathologist,
SRL Limited, Surat
Preanalytical phase is the most vulnerable part of the total testing process, and is considered to be among the greatest challenges to the laboratory professionals. However, preanalytical activities, management of unsuitable specimens, and reporting policies are not fully standardized nor harmonized worldwide.
Several standards related to blood sampling and sample transportation and handling are available, but compliance to those guidelines is low, especially outside the laboratory and if blood sampling is done without the direct supervision of the laboratory staff.
There is large heterogeneity in the criteria for sample rejection and the different strategies by which unacceptable samples are managed, processed, and test results are reported worldwide. Management of unacceptable specimens warrants, therefore, immediate harmonization.
Alongside the challenging and long road of patient safety, preanalytical phase offers room for improvement. Standardized blood collection method and using high-quality blood collection material in the form of syringes and needles plays an important role in quality laboratory reports.
Different companies give different types of syringes according to usage preference. Not only laboratories but also hospitals need syringe needles in different departments. Luer-Lok tip is generally used for injections requiring a secure connection of the syringe to another device.
Luer slip tip provides a friction-fit connection that requires you to push and twist the syringe tip into the needle hub. Eccentric Luer slip tip is generally used for venipunctures and aspirational fluids, allowing close proximity to the skin.
The catheter tip is used for flushing catheters, gastrostomy tubes and other devices like anesthesia needles and syringes. Cornwall fluid-dispensing syringe is designed for fluid transfer.
High-quality, easy-to-use injection devices designed to protect healthcare workers from needlestick injuries and exposure to bloodborne pathogens. These needles are available in a wide range of gauges, lengths, and bevel designs.
It includes both regular wall and thin wall offerings. Blunt fill and blunt filter needles are specifically designed to reduce the risk for needlestick injuries during medication preparation.
Selection of the best quality, according to budget and needs, can definitely help to improve the quality of laboratories.












