MRI Equipment
MR Evolving To Replace CT?
MRI has the potential to play an even bigger role in diagnosing and treating more conditions and diseases. The field is still wide open.
The last few years have seen significant advances in the field of MRI imaging. The quality and resolution of MRI have improved, primarily due to more powerful magnets. Electronics and imaging software have also improved as in other imaging technologies. At present, there is little that can be done to further improve the operation of the basic MRI system while significant limitations and challenges remain. Operating costs, installation complexity, and safety concerns need to be addressed. Scan time—arguably the most important factor in operating costs and patient concerns—has not improved in any meaningful way. Healthcare professionals are in agreement that shortening scan times while preserving image quality is the biggest game-changer.
Thanks to advances in technology and computing power, researchers and clinicians have found ways to speed-up scan times, including by making patients more comfortable and sampling less. In some cases, they have been able to reduce scans that previously took up to an hour to less than 10 minutes. The less time an adult or child has to spend in the MR scanner, the better.
Researchers and manufacturers have developed a number of different ways to achieve faster scans without sacrificing image quality. Some involve getting the patient to relax more. Others include improvements in the planning, scanning, and processing of exams, and some involve machine learning and neural networks to reduce the number of sequences required to create quality images of the organ or region of interest.
Advances in surface coil technology have also resulted in faster scan times. In general, the more channels a surface coil has, the more signal can be harvested, and the higher the resolution that can be delivered. Over the last year or so, the market is witnessing higher-channel coils coming. Most scanners use eight coils. Some have flexible coil options in 16 channels. Now the industry is witnessing 16- to 18-channel rigid coils become available. This advance gives a substantial boost, particularly if anyone coming from older technology with eight or fewer channels. New planning software that guides the technologist through prescribing image acquisition on the scanner is having an impact on scan time as well.
In addition, new computing capabilities have resulted in shorter scan times. Researchers have discovered that they are able to acquire under sampled data, saving significant scan time, and then use new, fast storage devices for memory extension to reconstruct clear and precise images. Another development that is helping to reduce MR scan times is the ability to postprocess scans with accelerated protocols.
Does it appear that with all these different approaches researchers are getting to the end? Have they reached the limit on minimum scan times? They do not think so.
There is no doubt that the market is witnessing a huge paradigm shift in the focus and design requirements for new MRI acquisitions, leading to the fast-paced development of new, fast MRI methods.
The field is still wide open. There is still a lot of potential for new algorithms and exploiting other characteristics of MR. There is a fundamental limit of signal-to-noise ratio. At some point, one has to sample long enough to register it. But every time researchers think they have had a lot of innovation in MRI and it is maturing, something new comes along. So, it is not feasible to say we are there. What’s even more exciting, is, if MR scan times can be reduced to no more than a few minutes, MR has the potential to play an even bigger role in diagnosing and treating more conditions and diseases. MR, which does not require ionizing radiation, could be a very interesting alternative for CT, especially when one combines it with Artificial Intelligence (AI) and adaptive algorithms.
Indian market
The Indian MRI equipment market for 2018, is estimated at Rs 1700 crore, and 346 units, by volume. It continues to be dominated by Siemens, GE, and Philips. Toshiba and Hitachi are the other aggressive brands.
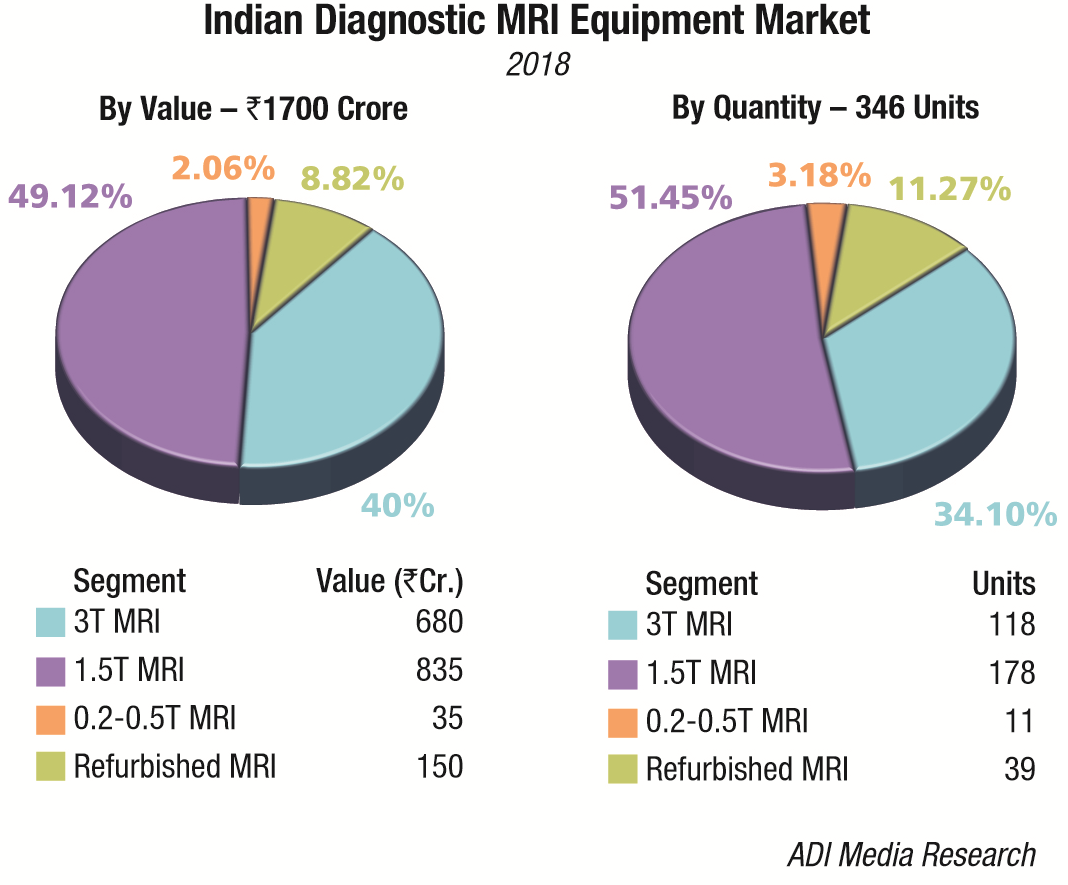
The premium systems 3T and 1.5T, continue to dominate the market with an approximate 89 percent share. The discerning customer continues his drive to upgrade the 1.5T systems to 3T MRI systems. The 7T systems continue to evade the Indian market. The 0.2-0.5T MRI machines and refurbished machines cater to the cost-conscious smaller hospitals all over the country.
The sector may soon see an indigenous MRI machine, which could be much cheaper than those made by Philips and GE. The government is working on the first Made in India MRI machine, the prototype of which is soon expected. Once that is ready, it will take around one more year to make it market-ready with all the required processes such as quality check and trial runs. The Minister of Electronics and Information Technology (MeitY), Ravi Shankar Prasad is pushing for a 2019 launch.
Market – 2018* |
|
|---|---|
| Tier I | Tier II |
| Siemens, GE, and Philips | Toshiba and Hitachi |
| *Vendors are placed in different tiers on the basis of their sales contribution to the overall revenues of the Indian MRI equipment market. | |
| ADI Media Research | |
In June 2018, Tata Trusts had launched a portable India-made MRI scanner, which was developed by VoxelGrids, a Bengaluru based MRI technology startup supported by Tata Trusts’ Foundation for Innovation and Social Entrepreneurship (FISE). The lightweight MRI scanner is the first of its kind in India. It can be mounted on a truck and transported to the remotest villages, where it can be switched on again at any primary health center to perform MRI scans on the patient’s body. The MRI scanner is expected to cut the cost of scanning by as much as 50 percent. This project of VoxelGrids and FISE, also known as Social Alpha, won the Indo-US Science and Technology Forum (IUSSTF) award in 2017, while it was still getting developed. Sri Sathya Sai Institute of Higher Medical Sciences of Bengaluru is the clinical partner of this initiative, where the first system has already been installed.
Global market
MRI systems are becoming more powerful, the quality of images is improving and the patient’s comfort is now taken largely into consideration. The global MRI market is projected to rise from a valuation of USD 7047 million in 2018 to reach USD 9722 million by end of 2023, reflecting a CAGR of 6.6 percent, predicts Transparency Market Research. Although the technology has improved, and purchases are being made toward the 3.0T product segment, 1.5T remains the fastest growing segment within the MRI market from 2018 through to 2023. Rapid growth is projected in emerging markets in countries within the Middle East and Africa. The Chinese market is estimated to have been the second largest market globally in 2018, due to its large population size and growing middle class, namely in regards to healthcare needs. Along with players becoming more prominent in the Chinese MRI equipment market, the Chinese aging population is resulting in more demand for diagnosis using imaging products including MRI.
The purchase of MRI systems to replace existing installs may be limited in the future by the ability of healthcare providers to simply upgrade the existing install, either replacing key components or advancing software. With healthcare spending remaining under scrutiny in a number of mature and emerging markets, this could provide a cost-effective option to improve the quality of care provided, whilst limiting the financial impact of the upgrade. This may limit the growth of the future installed base, the number of units shipped, and could lead to a further lengthening of replacement cycles of MRI systems.
Factors such as escalating aging population, technological advancements in MRI systems and growing awareness on the early diagnosis of diseases are supporting the growth of this market. Increasing life expectancy and falling death rates are the major contributors to the growth in the geriatric population. As per the United Nations Department of Economic and Social Affair (UNDESA) report World Population Aging 2017, the population of people aged 60 years or above is growing at a high rate. Furthermore, geriatric care is complex and requires better management through cutting-edge technology and treatment. Apart from general radiology, MRI offers a better characterization of most musculoskeletal diseases and is also used for suspected soft tissue mass or invasion. Thus, owing to the intensive care required for geriatric population, healthcare settings are increasingly adopting technologically advanced radiology systems, which, in turn, are boosting the growth of the MRI systems market, globally.
Furthermore, continuous R&D activities have led to various advancements in MRI systems in recent years. These advancements have further led to the evolution of MRI systems that offer improved image quality, better throughput, and faster exam time. Hybrid MRI and MRI-compatible devices are some of the advanced products available in the market.
Also, to facilitate the detection of multiple anomalies in different parts of the body with a single MRI scan, companies in the market are focusing on the development of multi-contract MRI scanners. For instance, in October 2017, Siemens AG received the US FDA approval for its 7T MRI system, Magnetom Terra. The system is recommended for patients weighing more than 66 pounds and is used for the examination of the head, arms, and legs (extremities). In July 2017, the US FDA granted marketing clearance to the Embrace Neonatal MRI System, the first MRI device specifically for neonatal brain and head imaging in neonatal intensive care units (NICU). The Embrace Neonatal MRI System, manufactured by Aspect Imaging Ltd, is designed specifically for imaging of the neonatal head. The Embrace Neonatal MRI System may be used on neonates with a head circumference up to 38 centimeters and weight between 1 and 4.5 kilograms. The system has a temperature-controlled incubator placed directly into the MRI system, minimizing movement of the baby. If urgent access to the baby is necessary during the imaging process, the baby can typically be removed from the system in less than 30 seconds. Thus, advancements in technology are leading to the introduction of more efficient and compatible MRI systems, which, in turn, is driving the market growth.
The MRI systems global market is dominated by players, including Philips N.V., Siemens AG, General Electric Company, Hitachi Ltd., and Canon Medical Systems Corporation. These market players offer a wide product portfolio and also have an extensive distribution network.
Some of the other companies operating in the MRI systems industry are Esaote S.p.A, Fonar Corporation, Shenzhen Mindray Bio-Medical Electronics Co. Ltd., Neusoft Medical Systems Co. Ltd., Aurora Healthcare US Corp., Bruker Corporation, Time Medical Holdings, and Aspect Imaging Ltd.
Vendor update
In February 2018, U.S. Food and Drug Administration (FDA) cleared Canon Medical Systems’ Vantage Galan 3.0T XGO Edition MRI. Outfitted with the all-new Saturn X Gradient, the system can provide up to 30 percent improved signal-to-noise ratio (SNR) for brain diffusion-weighted imaging (DWI), resulting in even higher resolution neuro images than previously offered. The system enables sequences for quantitative analysis and allows cardiac exams to be completed with fewer breath holds and improved patient comfort.
In June 2018, Elekta announced that its Elekta Unity magnetic resonance radiation therapy (MR/RT) system has received CE mark, clearing the technology for commercial sales and clinical use in Europe. Unity has the potential to transform how clinicians treat cancer by enabling the delivery of the radiation dose while simultaneously visualizing the tumor and surrounding healthy tissue with high-quality MR images. Unity also integrates advanced tools that allow clinicians to adapt the patient’s treatment to this current anatomical information.
In June 2018, Zetta Medical Technologies announced the release of Zoom, its latest MRI software algorithm for image quality enhancement and image optimization of short scanning techniques. Zoom is vendor-neutral and works with all MRI models from all major manufacturers. Its core algorithm was designed to help MRI imaging departments to automatically process all MRI imaging techniques, including time-sensitive short scan. The algorithm utilizes standard DICOM communications protocol to receive data, processes it and automatically transfers the enhanced images to the picture archiving and communication system (PACS). Its engine can simultaneously manage incoming data from multiple scanners to satisfy aggressive workflow demands. Developed in the U.S. by Zetta, Zoom helps MRI imaging departments maintain patient care, increase patient throughput and improve scanning profitability.
In September 2018, Philips announced the launch of the Ingenia Ambition X 1.5T MRI scanner. It is the latest advance in the Ingenia MRI portfolio, which comprises fully digital MRI systems, healthcare informatics and a range of maintenance and life cycle services for integrated solutions that empower a faster, smarter and simpler path to enabling a confident diagnosis. The first commercial installation of the Ingenia Ambition X was recently completed at Spital Uster Hospital, Zurich, Switzerland. The Ingenia Ambition X is CE marked and has received 510(k) clearance from the US FDA.
In October 2018, GE Healthcare signed a memorandum of understanding (MoU) with SAMEER, a research and development (R&D) lab under the Department of Electronics and Information Technology (MeitY), to co-develop an indigenous MRI machine. GE Healthcare and SAMEER will collaborate on the research, design, and development of a 1.5 Tesla MRI platform that will give rise to whole-body, portable, and digital MRI machines. The multinational firm has supplied the magnet, which is one of the most critical components, for the prototype MRI machine that SAMEER is developing. Apart from working with SAMEER, GE Healthcare, through its joint venture partnership with Wipro in India, is also looking to grow skilling to support the government’s Ayushman Bharat universal healthcare scheme. The company expects demand for its devices to go up as healthcare becomes more accessible, and is looking to train people who can operate these machines.
In October 2018, the US FDA cleared the Magnetom Sola, a 1.5T MRI scanner from Siemens Healthineers that brings Siemens’ BioMatrix technology to the 1.5T market. This technology addresses patient anatomical and physiological differences, as well as differences in how users set up and conduct MRI exams, to increase productivity and decrease rescans for improved efficiency and patient satisfaction. The Magnetom Sola helps healthcare providers perform a full range of routine and complex MRI exams while accelerating workflow and delivering consistent results across all patient types. BioMatrix Sensors save setup time and inform the correct exam strategy. Respiratory Sensors in the patient table eliminate the need for navigators with respiratory-triggered sequences. A new kinetic sensor, an in-bore camera system, enables technologists to visually monitor the patient’s face. BioMatrix tuners improve the quality and reproducibility of head, neck and spine imaging using distortion-mitigating software and hardware. BioMatrix Interfaces utilize AI and body models to expedite patient positioning and deliver consistent, reproducible results.
Research update
Scientists develop new MRI tool for cancer diagnosis and therapy. A European research group has developed a system that allows doctors to both improve the accuracy of diagnosing malignant cells and to provide additional opportunities for cancer treatment. The magnetoferritin compound is the main element of the new system. The research article has been published in Advanced Functional Materials. The research team consists of scientists from the National University of Science and Technology (NUST) MISIS (Moscow), the Technical University of Munich, Helmholtz Zentrum München, the University of Duisburg-Essen, and the University of Oldenburg.
The lack of accuracy (contrast) in imaging is a common problem of non-invasive diagnosis. Contrast agents, compounds that are introduced into the body before a diagnostic procedure to enhance the response and make affected cells more visible on a tomograph, can be used to solve this problem in MRI. Paramagnetic gadolinium particles and superparamagnetic iron particles are among these agents. However, even in small quantities, these substances – alien to the human body – can potentially be dangerous.
“The international research team, including Dr Ulf Wiedwald, a visiting professor at the NUST MISIS Biomedical Nanomaterials Laboratory, has developed a unique injection diagnosis system based on magnetoferritin. The developed system will significantly improve the quality of MRIs and optical diagnosis,” said Alevtina Chernikova, rector of NUST MISIS.
Magnetoferritin is a compound consisting of endogenous human protein (ferritin) and a magnetic nucleus. The development and testing of the compound were conducted following the existing protocol for the synthesis of magnetoferritin, but was improved for the effective capture of tumor cells. The high concentration of magnetoferritin in tumor tissue made it possible to obtain a hypoallergenic contrast agent that is perfectly compatible with the human body.
“An intravenous injection of magnetoferritin has been proposed. Then, spreading with the blood flow, [the magnetoferritin] will be captured by the targeted tumor cells. As has been shown in a large number of studies, these cells actively capture transferrin – the protein responsible for the transport of iron in the blood. The same receptors are capable of capturing the magnetoferritin as well. Once they get into the lysosomes of targeted cells, the magnetoferritin will further enhance the contrast signal,” commented Wiedwald.
The system will also allow doctors to conduct therapy on tumor formations. If an MRI shows cancerous cells, they can be targeted by an electromagnetic field or light, which will lead to their heating and subsequent death.
AI provides faster, clearer MRI scans. A research team with funding from the National Institute for Biomedical Imaging and Bioengineering (NIBIB) has developed an advanced computing technique for rapidly and cost-effectively improving the quality of biomedical imaging. The technology, called Automap, uses machine learning and software, referred to as neural networks — inspired by the brain’s ability to process information and perceive or make choices. Automap finds the best computational strategies to produce clear, accurate images for various types of medical scans.
In their study in the March 21, 2018, issue of Nature, the researchers from Massachusetts General Hospital (MGH) Martinos Center for Biomedical Imaging and Harvard University found that the Automap system could produce brain magnetic resonance imaging (MRI) images with better signal and less noise than conventional MRI techniques. Achieving a good signal-to-noise ratio is a key factor in generating a quality MRI scan.
“The signal-to-noise ratio improvements we gain from this AI-based method directly accelerates image acquisition on low-field MRI,” said lead author Bo Zhu, Ph.D., a postdoctoral research fellow in radiology at Harvard Medical School and in physics at the MGH Martinos Center. NIBIB has supported Zhu’s postdoctoral research on this project. He added that the Automap neural network will be compatible with novel image acquisition strategies and unconventional hardware designs.
Automap churns through – and learns from – data from existing images and applies mathematical approaches in reconstructing new ones. The team used a set of 50,000 MRI brain scans from the NIH-supported Human Connectome Project to train the AUTOMAP system to reconstruct images in their study, successfully demonstrating improvements in reducing noise and reconstruction artifacts over existing methods.
Automap achieves almost instantaneous image reconstruction, according to senior author Matt Rosen, Ph.D., director of the Low-field MRI and Hyperpolarized Media Laboratory and co-director of the Center for Machine Learning at the MGH Martinos Center. The reason for the rapid processing speed — just tens of milliseconds — is that the neural network has no cycles or loops, rather is a feedforward system.
“Some types of scans currently require time-consuming computational processing to reconstruct the images,” Rosen said. “In those cases, immediate feedback is not available during initial imaging, and a repeat study may be required to better identify a suspected abnormality. Automap would provide instant image reconstruction to inform the decision-making process during scanning and could prevent the need for additional patient visits.”
“This technology could become a game changer, as mainstream approaches to improving the signal-to-noise ratio rely heavily on expensive MRI hardware or on prolonged scan times,” said Shumin Wang, Ph.D., director of the NIBIB program in MRI. “It may also be advantageous for other significant MRI applications that have been plagued by low signal-to-noise ratio for decades, such as multi-nuclear spectroscopy.”
Looking ahead
Researchers are currently pushing the boundaries of what the technology can or should be doing, but whatever they come up with will have to undergo extreme vetting (validation process, peer review, etc). Also, potential benefits and the potential limitations need to be best determined.
Second Opinion
Cardiac MRI, The Huge Demand-Supply Mismatch
Dr Mona Bhatia
Director and Head of Department,
Department of Radiodiagnosis and Imaging
Fortis Escorts Heart Institute
Cardiac MRI is one of the fastest growing fields of cardiac imaging. India has a huge cardiovascular disease burden which exceeds all global averages and in particular infections, myocarditis, cardiomyopathies, hypertensive heart disease exceed global numbers besides the ever talked about ischemic heart disease. The growing promise of cardiac MRI in terms of diagnosis, prognosis, treatment, and follow up is making cardiac MRI the gold standard. Further, there are growing numbers of pediatric cardiac MRIs for congenital heart disease to reduce the radiation exposure from recurrent scans and lifelong follow up.
The last decade has seen a mere ten to fifteen-fold increase in cardiac MRIs, however, on a countrywide scale, the numbers of cardiac MRI are south of 20,000. India with a population of 1.3 billion has an approximate 6.5 million echocardiographic examinations annually. With growing interest and demand for cardiac MRI and assuming 1 percent of cases undergoing echocardiography could benefit from cardiac MRI, we could expect an exponential rise in cardiac MRIs. Needless to say, the capability and availability of cardiac MRIs are significantly lagging given the number of sites and trained personnel that would be needed to handle the volumes this new demand entails.
There is a growing need for high-end cardiac MR imaging to overcome the challenges faced in efficiency and throughput of these time and labor intensive studies. Lack of training and reduced efficiency with long scanning times were huge deterrents for cardiac MRI in busy departments. However, with moves to 3T, high-resolution, faster scanning protocols, and shorter breath holding without compromising image quality are now changing the vision towards the viability of cardiac MRIs even in busy departments. Given the huge need to have an accurate diagnosis and evaluation of the myocardium in diverse clinical settings, without doubt, India could expect exponential growth in the field of high-resolution cardiac MRIs.












