Life Sciences
MS opens up futuristic opportunities for rapid and deployable diagnostics
MS-based techniques are being explored as promising avenues in the study and detection of emerging pathogen outbreaks such as COVID-19.
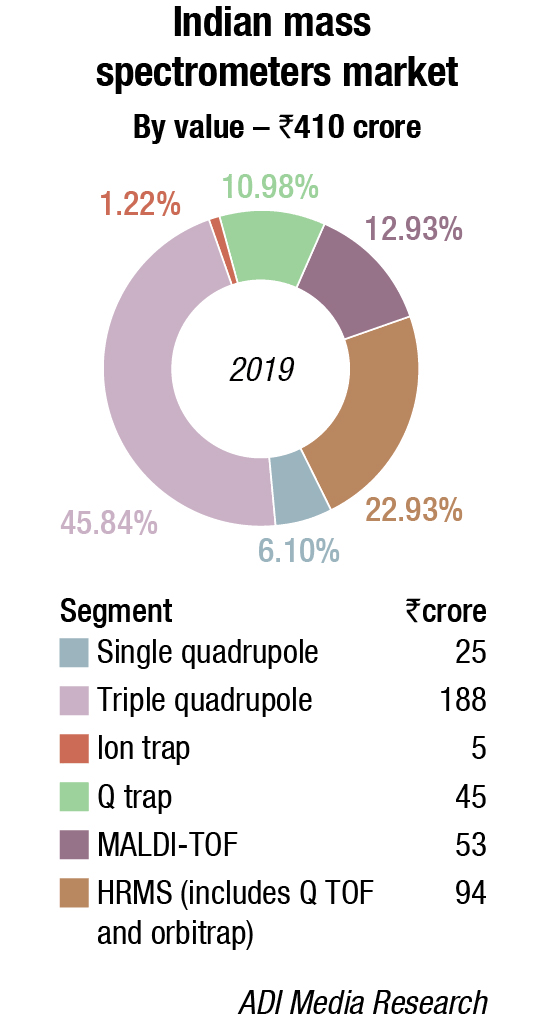 Mass spectrometry is one of the most dominant analytical techniques for both quantitative and qualitative applications. The last two decades have witnessed a continuous improvement in the MS techniques, facilitating the establishment and advancement of high-throughput, qualitative and quantitative analytical methods. A hike in implementation of hyphenated MS technologies, such as gas chromatography MS (GC-MS), liquid chromatography MS (LC-MS), tandem MS (MS/MS, GC-MS/MS, LC-MS/MS), and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) has made it a robust technique of this century.
Mass spectrometry is one of the most dominant analytical techniques for both quantitative and qualitative applications. The last two decades have witnessed a continuous improvement in the MS techniques, facilitating the establishment and advancement of high-throughput, qualitative and quantitative analytical methods. A hike in implementation of hyphenated MS technologies, such as gas chromatography MS (GC-MS), liquid chromatography MS (LC-MS), tandem MS (MS/MS, GC-MS/MS, LC-MS/MS), and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) has made it a robust technique of this century.
In the recent years, MALDI-MS became a routine laboratory technique for the identification of enriched bacterial and fungal cultures. This is one of the most prominent clinical applications of MS. Its shorter identification time, compared to conventional techniques, makes mass spectrometry a game changer in microbiology labs. Use of MALDI-ToF MS has further revolutionized the workflow for identification of microorganisms.
Paper spray ionization mass spectrometry (PSI-MS) has recently opened new opportunities for future clinical investigation to diagnose pathogens. Another rapidly emerging technique, PCR-MS is being exploited for its capability to directly identify known pathogens from the clinical specimens. Furthermore, miniaturized portable MS equipment broaden the frontier for possible applications with relatively fast and highly sensitive ways to analyze for viral compounds. As of now, identification of a subset of microorganisms has been cleared by FDA but the recent pandemic may propel the application of MS in detection of more viruses and microbes.
Current COVID-19 tests
The early part of the coronavirus global pandemic revealed weaknesses in traditional sophisticated infectious disease testing and inconsistency in the supply chain for instruments and reagents. This created avenues for development of assays that are more simplified and do not require multiple consumable reagents for the process-extraction, purification, amplification, and detection. It could substantially improve the testing capabilities of clinical laboratories.
 Dr Tasleem Raza
Dr Tasleem Raza
Professor & Incharge, Central Research Lab, Department of Biochemistry, Era’s Lucknow Medical College
“Mass spectrometry is a powerful analytical technique which has been witnessed to gain profitable growth avenue in market owing up to USD 13.98 billion by 2026 registering a CAGR of 9.2 percent. It is attributed to impede lucrative growth trend in pharmaceutical industry and research organizations.”
MALDI-MS has emerged as one such technology with the potential to require minimal reagents for testing. Recently, a publication in Clinical Chemistry reported the development of a MALDI-MS method for the diagnosis of SARS-CoV-2 infection. Nasal swab samples were directly analysed with MALDI-MS and a spectral pattern was used to classify infected and non-infected patients. The study reports high accuracy (93.9%) with 7 percent false positives and 5 percent false negatives. There are multiple desirable features in the assay protocol for a clinical test during the COVID-19 pandemic, including minimal specimen preparation, few reagents, flexible sample throughput, and rapid data acquisition.
This rapid method can give results in minutes and requires minimal consumables or reagents, unlike traditional biochemical or molecular microbiology techniques. The MALDI-MS instrument is the major expense in this test. It is an important caveat as the upfront capital investment of MALDI-MS is currently cost prohibitive for laboratories in many countries. In general, MALDI-MS appeals to clinical laboratories based in low resource settings or in distant geographic locations where the supply chains for reagents is not robust. But a more widespread application of this method for COVID-19 testing may not prove to be as feasible.
MAP Sciences, along with a University of Cambridge Laboratory, have come up with a rapid and cheap gargle test as a solution to Covid-19 testing challenge. In this, mass spectrometric analysis is used for identification of SARS-CoV-2 proteins in gargle solution samples of COVID-19 patients. The sample is taken by simply gargling with 10ml of tap water – a welcome alternative to the nose and throat swabs of the existing tests. With an under one-hour turnaround time, the developers claim the test to be quicker, easier, cheaper and as accurate as current PCR testing. The assay itself might cost a lab as little as £2.48 per sample.
MS-based COVID-19 test developed by Indian team
Researchers from the Institute of Genomics and Integrative Biology (IGIB) and the National Centre for Disease Control (NCDC) have developed a technique that uses mass spectrometry to detect SARS-CoV-2 virus. The MS based technique relies on detection of the presence of two peptides which are unique to SARS-CoV-2 virus.
The new method can directly identify the virus without amplifying the RNA for detection, as is the case with the current gold standard RT-PCR test for detection of novel coronavirus infection.
The developers reported that the peptides of SARS-CoV-2 virus can be even detected in patients who have recovered from the symptoms and have tested negative for the virus by RT-PCR. The targeted peptides remain present even after 14 days of initial infection.
They also reported 95 percent sensitivity and 100 percent specificity of this new method with respect to RT-PCR, which is much better than the alternative rapid antigen kits. The rapid kits tests are currently being used in India for scaling up testing but throw up 20 to 50 percent false negatives.
With less than three minutes detection time, the entire process from sample preparation to detection would take less than 30 minutes. Whereas, the RT-PCR test takes a minimum of 2-5 hours including time taken for a sample transportation. Evidently, the mass spectrometer equipment is expensive, but it would cost only about Rs.100 for each test, which makes it cheaper than RT-PCR, and many research labs already have the mass spectrometer.
Future perspective
It is widely acknowledged that there is a need for rapid, sensitive, and specific diagnosis of SARS-CoV-2 by fast and unambiguous testing methods in response to the current outbreak. As part of future preparations for pandemic, the use of alternative technology such as MALDI-MS has great potential for indirect detection of infections. Increasing production of portable MS can be very important for clinical lab adoption. It will not only help overcome the challenges of high cost or requirement of a significant lab footprint to operate, but also augment the potential for remote testing and screening. Moreover, improvement in performance and sensitivity of miniaturized MS can widen the potential span of applications, including in disease outbreaks.











