Thermal Cyclers
PCR – Then, now, and next
Despite its importance over the past year, PCR advancements are not limited to COVID-19 testing. The technology is continually improving, and researchers are striving to reduce the cost and complexity of these reactions.
In the wake of rapidly increasing coronavirus cases, the demand for testing kits surged. In India, the number of tests for the detection of COVID-19 exceeded the 61.39-crore mark as of November 6, 2021.
India expanded from 14 laboratories capable of conducting COVID-19 tests in February 2020 to more than 1500 over the next six months. The country now has nearly 3000 such labs. Amongst all the testing mechanisms, which are currently employed, PCR remains the most prevalent. The test is being extensively employed to detect the presence of the virus and to determine the degree of infection within the patient.
A high degree of accuracy and reliability of testing data is what makes the RT-PCR approach highly popular amongst medical practitioners to detect COVID-19 cases. The testing typically takes 4 to 8 hours while the results are delivered almost 24 hours after the testing has been conducted. Due to this time lag, healthcare settings are looking for alternative testing mechanisms capable of delivering the results in a shorter timeframe.
Nevertheless, the RT-PCR testing is projected to maintain an upward sloping curve until the time the pandemic is there. Additionally, home testing kits are being rolled out to facilitate unfettered access to rapid and safe COVID-19 testing.
The consumables and reagents segment dominated the market and accounted for the largest revenue share of more than 69 percent in 2020. This growth is attributable to a substantial demand for testing supplies for the SARS-CoV-2 pandemic and the increased availability of technologically advanced diagnostic equipment in countries with unmet clinical needs. In addition, the launch of new products contributed to the growth of the segment. For instance, in March 2021, PCR Biosystems announced the launch of its IsoFast Bst polymerase reagents that support sensitive, robust, and rapid amplification of RNA and DNA, which enables faster testing procedures.
The global PCR machines market is estimated at USD 1545.2 million in 2020. Over the next five years, it is expected that the PCR machines will register a 5.7-percent CAGR in terms of revenue, and the market will reach USD 1929.2 million by 2026.
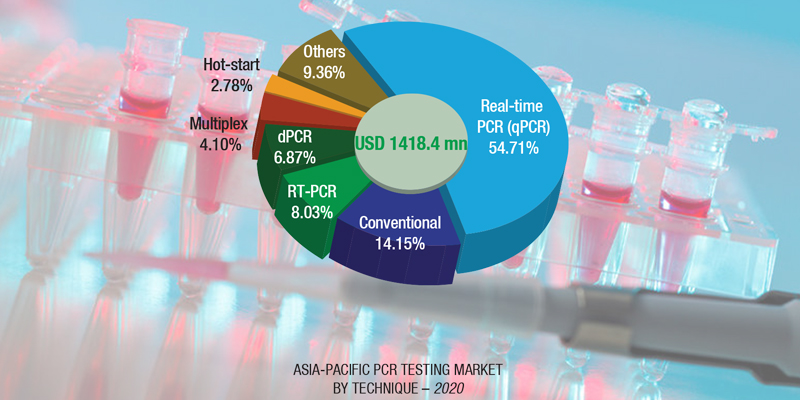
The PCR technologies market, amid the COVID-19 pandemic, experienced positive growth in terms of COVID-19 testing due to the positioning of PCR as the gold standard for genomic sequencing/identification (and hence is the preferred choice for screening). This resulted in significant industry focus toward the early commercialization of PCR-based COVID-19 diagnostic tools. However, since PCR is also a preferred technique for cancer screening, its demand in cancer screening applications reported a sharp decline in 2020, owing to restricted public movement and de-prioritization of non-essential medical services by respective governments worldwide.
The technology is improving and is shifting toward easy-to-use products. For instance, MatMaCorp’s handheld PCR device MYRTA was launched in May 2021, facilitating real-time detection. A new entrant diagnostic system in the segment is the QuantStudio 5 Dx RT-PCR system, which was introduced by Thermo Fisher Scientific in March 2021 for laboratory use as well as assay development. The launch of the system was aimed at meeting the rising demand for PCR systems globally. In addition, the cost-effectiveness and easy-to-use interface of the system is anticipated to fuel segment growth.
Emerging markets are expected to offer significant growth opportunities, led by the rising incidence of infectious and chronic diseases, as well as increasing R&D initiatives to develop innovative genomic techniques such as PCR. Growth will be supported by the expansion of healthcare infrastructure, increase in healthcare expenditure, and reducing procedural costs for qPCR-based and dPCR-based disease diagnosis in emerging countries.
Some of the leading players include Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., Eppendorf AG, Fluotics, Hamilton Company, Illumina, Inc., Integra LifeScience Holdings Corporation, MGI Tech Co., Ltd., Mascon, Inc., Qiagen Inc., Roche Diagnostics, Thermo Fisher Scientific, Thomas Scientific, and VWR International.
Since 1993, following the development of a method for monitoring PCR kinetics in real time, PCR techniques became fully quantitative (qPCR). These modifications of the basic PCR technique quickly became the gold standard for quantitative analysis of nucleic acids. Until now, the industry had three basic systems – end-point PCR, qPCR, and digital PCR (dPCR). Now there are many other variants of basic PCR techniques, one of which is bridge PCR.
The implementation of microfluidic systems led to the development of an entirely new family of devices, such as flow-through miniaturized and fast PCR, first using a space-domain system as seen in the very early cyclers. Researchers involved in silicon micromachining quickly took up this challenge, developing miniaturized and portable end-point PCR and then qPCR devices.
Digital PCR (dPCR). Many technologies evolved from original PCR – a prominent being dPCR, which is based on splitting a PCR sample into thousands, in some cases millions, of subsamples from the original to digitize the pool of DNA molecules, having either a single or no copy in each subsample. dPCR is always based on microfluidics and it is either droplet-based or chip-based. dPCR is capable of determining the absolute quantification of the DNA/RNA copy number, avoiding time-consuming quantification by qPCR, based on standard curves. It also allows multiplex PCR to amplify the number of copies of DNA samples with unfavorable ratios between abundant and rare DNAs.
Droplet-based digital PCR (ddPCR). Compared with qPCR, ddPCR has the advantages of higher precision and a lower coefficient of variation in absolute quantification. The PCR multiplexing is typically conducted by probe-based fluorescence with different excitation colors for each DNA/RNA. It is also used to generate libraries for single-cell RNA sequencing.
Chip-based digital PCR (cdPCR). The sample is loaded into silicon chips with wells made by a micromachining technique. Then the thermal cycling is performed and the chip is imaged by fluorescence microscopy to determine the number of wells with positive PCR results. Multiplexing is also performed in the same way as in ddPCR. The silicon-based microfabrication allows the combination of cdPCR integrated with a heater/sensor, using an ion-sensitive field-effect transistor in each well to monitor the PCR, eliminating the need for a separate fluorescence imaging system.
Isothermal amplification. qPCR is conducted by thermal cycling and its rate is typically limited by sample cooling, which slows with increased sample volumes. The commercial utilization of PCR was limited for several years by patent protection granted to Cetus Corporation. Researchers tried to overcome problems related to cooling, and bypassing the patent protection by creating new nucleic acid amplification methods. The isothermal amplification overcomes the cooling problems because it requires no cooling process. Several techniques were developed with isothermal amplification, such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification, which are currently popular.
Outstanding challenges in PCR. Despite the great diagnostic potential of PCR, the success of each of its practical applications is highly dependent on the quality of samples containing nucleic acids for amplification. The spectrum of different inhibitors of PCR complicating the work in real-world samples, such as false-negative results or higher limits of detection, has been characterized. Procedures leading to sufficient purification of samples and to the development of inhibition-resistant DNA polymerases have been developed. It has been documented that dPCR is more resistant to the presence of inhibitors. Additionally, the successful PCR amplification of GC-rich DNA sequences represents another challenge. It is complicated by the generation of secondary structures hindering full denaturation and primer annealing, and represents multiple techniques to overcome this problem have been established.
The miniaturization of PCR devices highlighted another challenge, which should be solved pre-analytically to achieve sensitivity comparable to established laboratories. Miniaturized devices work with low-volume samples and, therefore, the real-world biological samples should be effectively isolated and pre-concentrated, respectively, prior to analysis with such devices.











