Biochemistry Instruments and Reagents
Redefining the role of biochemistry labs
Beyond new and improved assays, innovation is underway in how biochemistry connects through automation to other areas of clinical laboratories.
Biochemistry is rapidly expanding, becoming one of the most influential areas of science. Combining the core tenets of biology and chemistry, the field plays a huge role in the development of novel new scientific approaches. The implications of uncovering the causes of pathologies on a cellular level are huge. By being able to call on a working knowledge of biochemistry and other related disciplines, such as molecular biology and immunology, those working in medical science have the potential to transform global healthcare.
 Indian market dynamics
Indian market dynamics
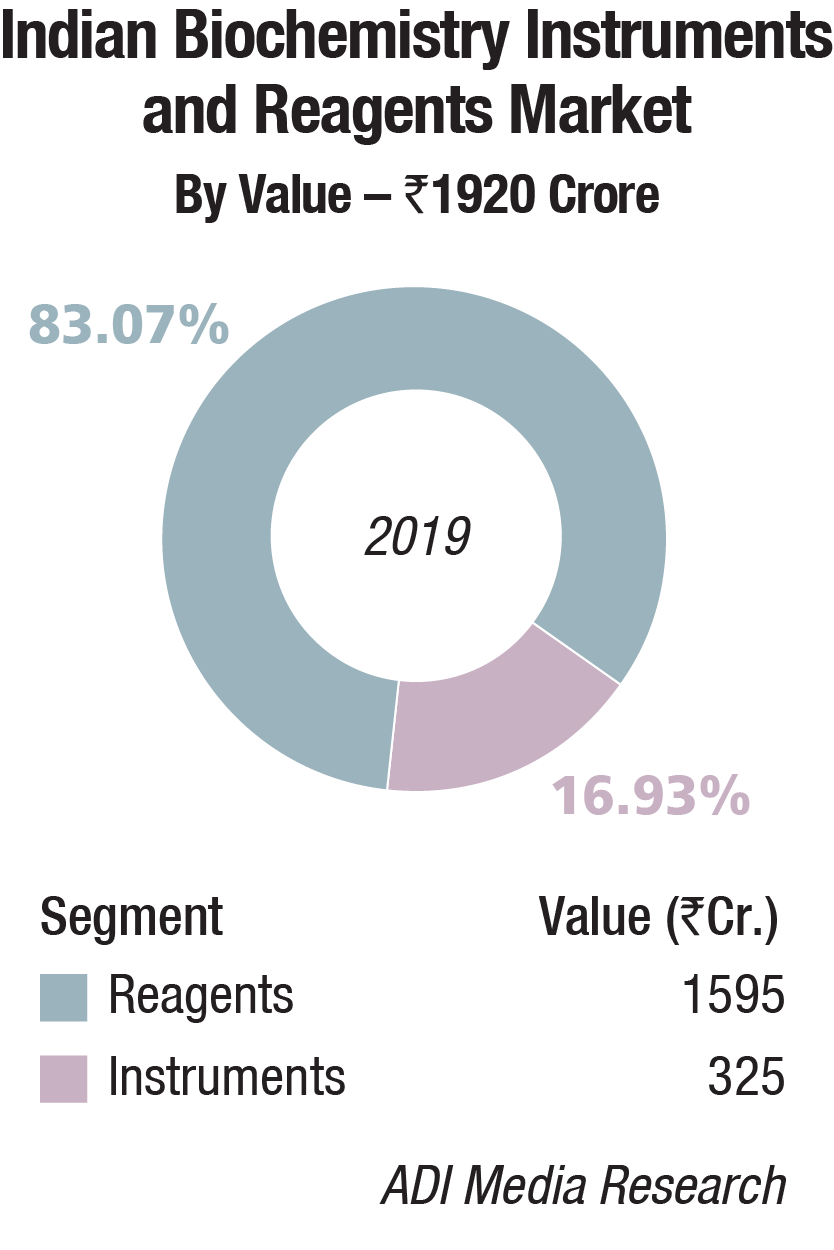
In 2019, the Indian biochemistry instruments and reagents market is estimated at Rs 1920 crore, with reagents continuing to dominate at Rs 1595 crore, at an 83 percent market share.
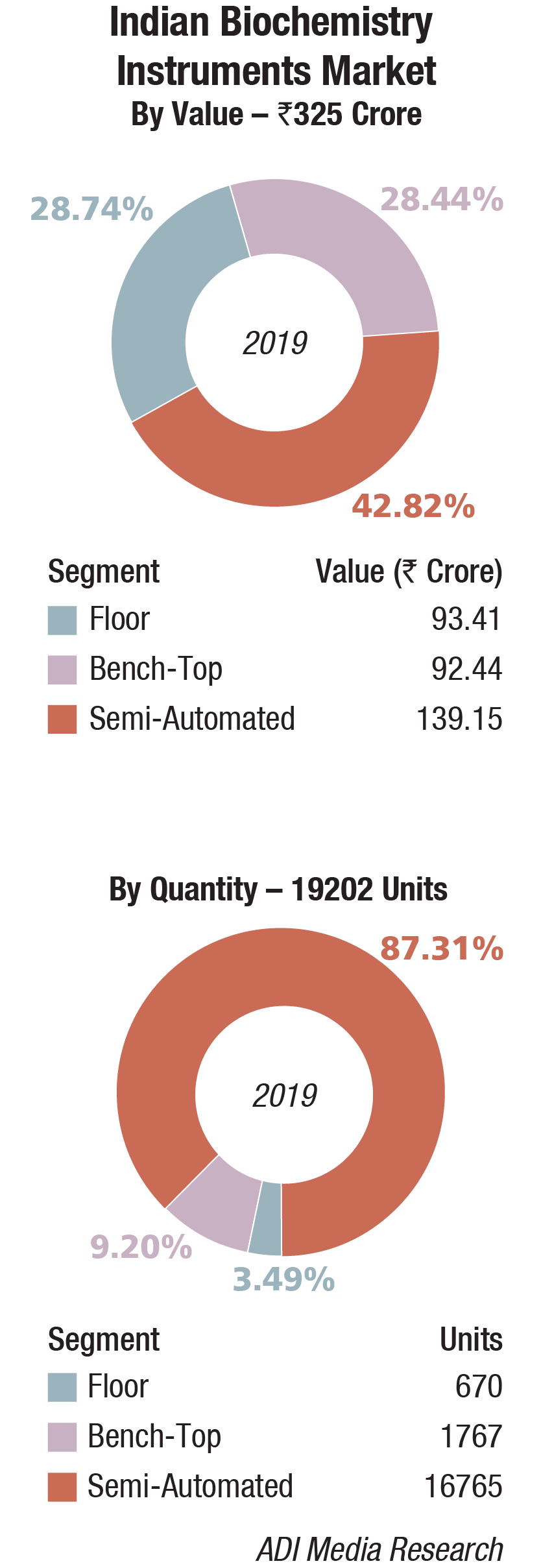
The floor-standing analyzers are estimated at Rs 93.41 crore and 670 units; benchtop analyzers at Rs 92.44 crore and 1767 units; and semi-automated analyzers at Rs 139.15 crore and 16,765 units. Almost 80 percent of floor instruments are on rentals; this figure is much smaller for bench-top. Semi-automated instruments are all procured, with almost none on rentals. The size of the market in 2019 has been calculated on assigning a monetary value to all the instruments installed, whether placed or sold.
As is the trend for hospitals, laboratories are also expected to see consolidation, thus impacting demand. With Reliance Life Sciences having announced its plans to enter the diagnostic sector, the disruptor that the group has a reputation for being, price erosion becomes a major possibility.
 Global market
Global market
The global biochemistry analyzers market was valued at USD 3.3 billion in 2018 and is expected to witness a robust CAGR of 5.4 percent over the next 6 years. Widespread advancements in the medical field have primarily been responsible for driving the global biochemistry analyzers market. Moreover, with rising geriatric population, the numbers of health issues are gradually increasing, thereby increasing demand for relevant treatments that involve biochemical analyzers.
Advanced analyzers are expected to boost market revenue. Conventional biochemistry analyzers that are used for repetitive analysis require large amounts of reagents and time. However, the technologically advanced analyzers are capable of automating the process of repetitive sample analysis that were earlier done by lab technicians. Industry players are developing biochemistry analyzers with the feature of positive identification that decreases the process of repeated pathogen testing. This feature is important for samples with low volume, such as neonatal units. Fully automated biochemistry analyzers can perform functions, such as recognition of sample and reagent bottles, cap piercing, tube sampling, dilution, and automatic re-run, which reduces human effort and time, and require lesser volumes of reagent and sample. These analyzers aid scientists in measuring the concentration of any substance in a reaction mixture. Therefore, the implementation of IT and automation in these analyzers is expected to drive the global biochemistry analyzers market growth. Furthermore, integrated systems that combine immunochemistry and biochemistry tests are gaining huge attention. It increases the workflow efficiency and delivers fast turnaround and high throughput. It also helps in achieving increased instrument capacity by connecting different analyzer units with a single sample-presentation mechanism. These systems are offered by manufacturers, such as Roche, Siemens, Abbott, and Beckman Coulter.
Increasing R&D for low-cost analyzers is expected to drive market growth. Increasing number of samples to be analyzed is increasing the demand for faster analytical methods that are capable of providing better information for decision-making. The need for automated analyzers for environmental and industrial samples has increased the research for new and cost-effective strategies of automation and control of analytical systems. The widespread availability of open-source hardware, along with novel analytical methods, has opened the possibility of implementing standalone automated analytical systems at low cost. However, new regulatory requirements, such as RoHS, have added complexity to instrument designing. High cost of tests and shortage of highly qualified and trained personnel are other factors restraining growth of the biochemistry analyzers market.
Major Vendors in Indian Biochemistry Instruments Market – 2019** |
|||
|---|---|---|---|
| Tier I | Tier II | Tier III | Tier IV |
| Roche, Transasia, Siemens, and Beckman | Bio-Rad, Mindray, and CPC | Robonik*, OCD, Randox, and Agappe | Tulip*, Trivitron, Dirui (marketed by Iris), Sysmex, Biosystems, Rapid, Biosys, Beacon, Vector Biotek, Microlab, and many Chinese brands |
| * SA Instruments Only | |||
| **Vendors are placed in different tiers on the basis of contribution of estimated value of total instruments installed-sold and placed to the overall revenues of the Indian fully automatic biochemistry instruments market. | |||
| ADI Media Research | |||
Positive identification with shorter turnaround time. Manufacturers are developing biochemistry analyzers with multiplexing analyzers. This type of system with shorter turnaround time gives advantages of high clarity and result accuracy. The feature of positive identification helps acquire accurate results in shorter run time by avoiding the inclusion of too many targets. On the other hand, besides pathogen testing, biochemistry analyzers are used for drug monitoring, drug abuse detection, and many more applications. Due to such technological advancements in the field of professional diagnostics, the applications of biochemistry analyzers that were initially restricted to the detection of infectious diseases are now venturing into other areas as well. As a result of this technological evolution, the diagnostics tests are now witnessing a boost in their demand.
Technological advances
When it comes to test menus for clinical chemistry and related areas, several new assays and new biomarkers are in development. Certainly, there are new tests that are always innovating in terms of the core laboratory.
For example, high-sensitivity troponin assays for diagnosing myocardial infarction are now becoming available to laboratories in the United States. New biomarkers may soon be available for diagnosing and monitoring traumatic brain injury, and encouraging research is emerging for tests for Alzheimer’s disease.
In addition, multi-analytic markers related to immune response to infection, perhaps in conjunction with molecular techniques, could help diagnose and predict the severity of infectious diseases, such as the outcome of a patient presenting with the early signs of sepsis.
Promising research also is underway involving biomarkers for ischemic stroke and for kidney disease. There is never a shortage of researchers looking for new markers, especially in the world of immunoassays.
In addition to new assays, innovations are leading to improved performance of existing assays and in core laboratory instruments. Automated platforms are being designed to handle a wider variety of sample types and smaller sample volumes. Furthermore, radioactive and toxic components of many assays are being replaced with safer materials, and there are innovations in electrical technologies and biosensors.
Beyond new and improved assays, innovation is underway in how biochemistry connects through automation to other areas of clinical laboratories. This is a continuation of a trend that has been happening over the past few decades, as core laboratories have grown to encompass disciplines spanning chemistry, immunoassay, hematology, and hemostasis, among other categories. Going forward, more novel technologies once reserved for specialized settings, such as molecular/virology, may increasingly migrate into the core laboratory. Efforts are underway to connect both mass spectrometry and molecular diagnostics instruments to clinical chemistry system. If mass spectrometry could be connected to chemistry and immunoassay analyzers on the same platform, drugs of abuse testing could be done in real time, with samples moving directly from immunoassay screening to confirmatory testing.
Likewise, if molecular diagnostics instruments were to connect to core laboratories, chemistry and immunoassay testing could be combined for diagnosing and monitoring infectious diseases, with follow-up molecular confirmation using the same sample on the same track.
The risk of contamination for molecular diagnostics tests has been a barrier in the past, but companies are coming up with new ways to manage this risk.
Meanwhile, advances in information technology (IT) and data analysis are impacting every area of clinical laboratories, including clinical chemistry. Diagnostics companies have improved the IT components of their products, incorporating dashboards and access to real-time analytics.
It is no longer an afterthought, but an active component of most major diagnostic companies in the clinical laboratory space to offer IT solutions that provide better access to viewing data in a way that is meaningful.
With these tools, clinical chemists can use data to improve laboratory performance. For example, they can monitor turnaround time for STAT tests with color codes and alerts. They can review patient medians for drifts or shifts that might indicate a calibration issue. Clinical chemistry labs also are using laboratory-generated data in a research context, looking for patterns that may be predictive of health conditions or that might guide decisions on reflexive testing or add-on tests. Because healthcare professionals are used to working with a large number of analyses and substantive data sets, chemists in particular are in a good position to play that role and to participate in more [electronic medical record]-based patient care initiatives using all of that laboratory data.
Outlook
Looking further into the future, the trends that have allowed many clinical chemistry assays to move to the point of care could ultimately influence the size of core laboratories, as well. Automation systems and chemistry instrumentation, oddly enough, have gotten a little bit bigger over time. With advances in technology and microfluidics, things should at some point start getting smaller.
Right now, most core laboratories are optimized for economies of scale and high-volume testing based on traditionally sized collection tubes, and cost per test on these platforms is much lower than on small devices at the point of care. Yet, if microfluidic technologies were to become less expensive, this calculation could change.
At some point in the future, the technology will catch up and things in a core clinical laboratory will start getting smaller, because a lot of labs are facing space pressures.
In the meantime, biochemistry innovation will remain largely in the realms of developing new biomarkers, improving existing technologies, and connecting biochemistry with other areas of clinical laboratories.
Industry Speak
 Dr Preet Kaur
Dr Preet Kaur
Business Unit Head-Biochemistry,
Transasia Bio-Medicals Ltd.
Choosing a biochemistry analyzer for your lab? Don’t miss out on the key attributes
With the shift toward offering value-based services, clinical laboratories are evaluated on the basis of high standards of quality and customer service. Laboratory automation can provide a solution to the quality demands and staff-shortage. However, only 5 percent of laboratories in India are fully automated. This is because they are often faced with the challenge of balancing cost with quality and patient safety. In this article, we focus on key attributes for selection of an appropriate semi- or fully automated biochemistry system. In the yesteryears, cost along with technical requirements was the most important criterion. However, with increasing emphasis on evidence-based medicine, quality and technical strengths are now important. Depending on the workload, a laboratory can opt for either a semi- or fully automated analyzer, based on various operational criteria, such as test throughput, method, continuous/batch or random-access mode, and reagent stability.
Quality control is another attribute that has gained a lot of importance in the clinical laboratory. The quality of a lab depends on its equipment and trained staff. The equipment should be able to generate results that can be reproduced and repeated in different diagnostic centers. While deciding on an equipment, one should consider the quality checks conducted on the analyzer, such as validation studies including analytical measurement range and method comparison. An instrument needs to be user-friendly, with minimum steps, and intuitive software for ease of operation. The analyzer should be able to interface with the laboratory’s LIS system.
After-sales service by the manufacturer is another important criterion that should not be overlooked. It is important to choose a manufacturer who has a dedicated after-sales network to ensure minimum downtime. One should be careful about some companies out sourcing the services through third parties, which sometimes is not seen as a good sign. Companies are also integrating remote technology in their analyzers for predictive maintenance and on-board inventory tracking. A laboratory needs to take into consideration all these attributes before finalizing a biochemistry analyzer.
Transasia Bio-Medicals Ltd. offers a complete range of sophisticated semi- and fully automated biochemistry analyzers and reagents, suitable for start-ups to large laboratories. Our Made in India range is integrated with remote-diagnosis technology, and backed by the largest after-sales and application-support network in the Indian IVD industry.
Industry Speak
Emma Callaghan
Marketing Executive,
Randox Laboratories Ltd.
H-FABP in cardiac surgery-associated acute kidney injury
 Cardiac surgery-associated acute kidney injury (CSA-AKI) is a well-recognized post-operative complication of cardiac surgery and is the second-most common cause of AKI in the intensive care unit, occurring in up to 30 percent of patients. Certain patient groups are more susceptible to CSA-AKI, and vulnerability can depend on age, sex, pre-existing cardiac dysfunction, pre-existing chronic kidney disease (CKD), previous surgery, or comorbidity. The pathogenesis of AKI involves multiple pathways, including hemodynamic, inflammatory, and nephrotoxic factors that overlap, eventually leading to kidney injury.
Cardiac surgery-associated acute kidney injury (CSA-AKI) is a well-recognized post-operative complication of cardiac surgery and is the second-most common cause of AKI in the intensive care unit, occurring in up to 30 percent of patients. Certain patient groups are more susceptible to CSA-AKI, and vulnerability can depend on age, sex, pre-existing cardiac dysfunction, pre-existing chronic kidney disease (CKD), previous surgery, or comorbidity. The pathogenesis of AKI involves multiple pathways, including hemodynamic, inflammatory, and nephrotoxic factors that overlap, eventually leading to kidney injury.
Fatty-acid-binding proteins (FABPs) are small cytoplasmic proteins that are abundantly expressed in tissues with an active fatty-acid metabolism. Primarily, their function involves the facilitation of intracellular long-chain fatty-acid transport. Heart-type fatty-acid-binding protein (H-FABP) is commonly utilized as a marker for myocardial ischemia as it is released 30 minutes following an ischemic attack, with concentrations peaking approximately 6–8 hours following chest pain onset. H-FABP measurement is useful in heart failure, CKD, diabetes mellites, and metabolic syndrome, all of which are important risk factors for post-operative AKI. The measurement of H-FABP pre- and post-cardiac surgery could identify those at a high risk of AKI and can assist in the development of an effective treatment plan, reducing mortality rates and the costs to health services.
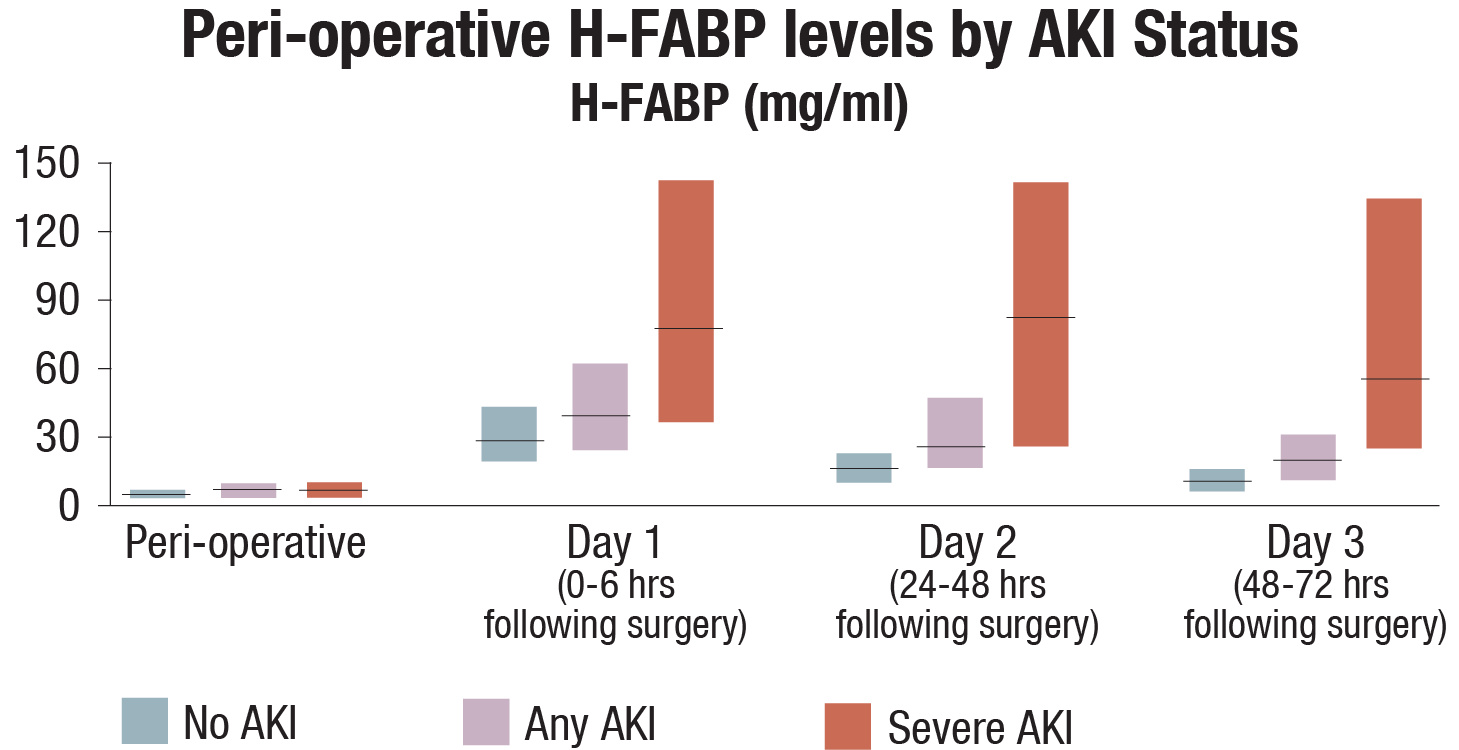
A study submitted to Kidney International (2015) evaluated the association between H-FABP and AKI by utilized data from the TRIBE-AKI cohort study; a multi-centered cohort of 1219 patients who underwent cardiac surgery and were at a high risk of developing AKI. The study found that the elevated peri-operative H-FABP levels were observed in patients who experienced any AKI, compared to those who did not experience AKI. The highest peri-operative H-FABP levels were observed in those with severe AKI (Figure). The researchers found that the first post-operative (Day 1) H-FABP levels in patients with severe AKI increased by 13-fold and an increase of 8-fold was observed for the same time point in patients who experienced any AKI.
Industry Speak
 Sanjaymon KR
Sanjaymon KR
GM-Business Development,
Agappe Diagnostics Ltd.
New momentum in clinical chemistry automation
The Indian market has become a red ocean for clinical chemistry automation, owing to the presence of many small and large players providing equipment in domestic as well as in the international markets. In the last decade, there were many players adding value to this market segment by bringing in alternative approaches. The lack of reliable methods to manage data, resources, and workflows created a shift toward adapting to commercial solutions by mostly all the laboratories. An innovation is the need of the hour in this market segment. Laboratories are in need of complying with global regulatory requirements to improve their processes.
Pathologists and technicians working in clinical and research laboratories need equipment that grant them access to an automated platform for blood-sample testing with superior precision and efficiency. The clinical chemistry automated analyzers market is growing at a higher rate, and witnessed a 50 percent increase in the number of units imported. The typical workflow of a laboratory is the key parameter, which will undergo transformation in the near future. Shorter turnaround time of laboratory operations would be the derived benefit for all laboratories that will adopt the instrumentations incorporated with the latest photometry-enhancing techniques. The major hospitals and chain laboratories in the country already have set goals and processes to achieve maximum efficiency in each and every department. The presence of R&D-based firms will surely help accelerate along the growth path for these hospitals and laboratories. The need of a dedicated workstation and software to program the laboratory process is being identified as an inevitable factor throughout the value chain, instead of spending their day repeating the monotonous task. Major factors that are contributing toward the growth of the market include the compulsion from the government to comply with the regulations, and the growing demand for the integrated healthcare systems. On top of that, laboratories try to differentiate by introducing extended assays within the routine chemistry profile tests. The most important is to know which analyzer to use, as different analyzers have different analysis time and reagents, which make these instruments highly sophisticated.
Equipment that perform both biochemistry and immunochemistry assays on the same system have proved to be the most economical in the last decade. Further development of the already-established equipment allows facilitated handling and increased capacity for blood samples, reagents, and consumables. An extra leap taken today will help the laboratories from investing more for upgradation in the near future as the emergence and rapid adoption of robotics is increasing day by day. R&D-based Indian manufacturers have come up with automation analyzers that can upgrade laboratories to perform more tests per reagent pack and more samples per run. These benefits are derived by incorporating the latest techniques in photometry, low volume reagents consumption, and optimization of onboard facilities for reagents, samples, and consumables.
Industry Speak
 Shobhit Jain
Shobhit Jain
Product Manager-Biochemistry,
Sysmex India Pvt. Ltd.
Emerging trend of automation in biochemistry
Indian IVD market has witnessed a prolific growth in the past few years. Biochemistry market in India is the second-largest IVD market with 25–30 percent market share. With technological advancements, there is a growing need for automation in the biochemistry segment. Laboratory automation (total lab automation, integration, and standalone automation) is a complex integration of robotics, computers, liquid handling, and numerous other technologies. The purpose of automation is to save time and improve performance through the elimination of human error.
There are three phases of laboratory testing:
- Pre-analytical (pre-testing phase) – It accounts for up to 70 percent of all mistakes made in laboratory diagnostics, most of which arise from problems in patient preparation, sample collection, transportation, and preparation for analysis and storage;
- Analytical (testing phase) – It occurs within the clinical pathology laboratory and is usually the result of operator or instrument error. Errors in the analytical phase are very important because they lead to inaccurate test results; and
- Post-analytical (post-testing or reporting phase) is the last phase of reporting, which includes transcription errors, report sent to incorrect location, report illegible, or report not sent.
Modern biochemistry analyzers use automated discrete systems, as opposed to batch-analysis instrumentation, which allows for an almost unlimited mix of analyses on a single instrument, combining routine clinical chemistry and immunoassay tests and fewer analyzers, requiring less floor space and greatly improved operational efficiency. Manual and semi-automated procedures are now relatively rare. Benefits of replacing manual procedures with automation include eliminating potentially error-prone manual procedures with automated processes, requiring minimal technician involvement, increasing productivity, decreasing TAT, improving safety, minimizing error, improving sample handling, and allowing practical reallocation of laboratory staff who are freed from manual tasks.
Industry Speak
 Gaurav Bhide
Gaurav Bhide
Product Manager, Hematology and Biochemistry,
Trivitron Healthcare Pvt. Ltd.
Advances in biochemistry instruments
Recent technological advancements have led to a tremendous progression in the field of medical sciences owing to development in the fields of biotechnology and biochemistry. New techniques are constantly being introduced leading to precise diagnosis, development of remarkable and medicinally valuable entities, instruments, and reagents. Advanced biochemistry reagents being developed today, now offer better and precise diagnosis at a lower turnaround time, with minimal wastage. In fact, advanced biochemistry instruments and reagents have great impact in the fields of health, food, agriculture, and environment.
Biochemistry analyzers have now become completely automatic, making almost negligible manual involvement. Complete automation of pre-analytical, analytical, and post-analytical tasks enables laboratories to perform more work using less labor and fewer resources. Consolidation of stand-alone modules and integration of multiple units into a single system has led to the evolution of a highly efficient workstations that perform a number of tests and are capable of running a number of assays simultaneously.
Fully robotic and completely automatic systems now work on the pre-analytic phase, taking care of several important steps including identification, sorting, centrifugation, and various post-analytic processes like storage and archiving. Dry chemistry analyzers are already in use for various biochemistry analysis. These systems offer advantages of reagent stability, less carryover, and negligible unwanted exposure.
Biochemistry analyzers have now evolved to become cloud-based systems. Compatibility with the cloud enables to interface the analyzer software with advanced informatics techniques like laboratory information system or hospital information system. This maps demographic, clinical, and ethnic details with obtained laboratory results, thus avoiding manual/typographic errors.
Trials are in progress to combine mass spectroscopy and advanced molecular diagnostics and leverage those into clinical chemistry systems. Present-day analyzers are efficient to detect molecular concentration in the body. Integrating mass spectrometry and immunoassay analyzers with these systems will make them even more efficient, and various pharmacokinetic and pharmacological aspects of drugs can be analyzed on a real-time basis.
We see that advances in biochemistry instruments are driven by a number of factors, which have led laboratories evolving to healthcare hubs with a number of integrated platforms, conducting hundreds of laboratory analysis per hour with advanced information-management systems. In future, the growth of a laboratory will rely on this foundation, enhancing capabilities of small or mid-level labs.
At Trivitron Healthcare, we have introduced fully automatic biochemistry analyzers, which are compact and suitable for all-sized laboratories, technologically advanced for today’s needs.
Industry Speak
 K Chandramohan
K Chandramohan
Product Manager,
BioSystems Diagnostics
Biochemistry analyzers gaining a technical edge
Biochemistry analyzers are becoming technologically advanced in terms of precision and speed. The accuracy of biochemistry analyzers in analyzing urine and blood samples has benefited diagnostic centers and pathology labs in recent years.
Market dynamics
The major factors driving biochemistry analyzers market growth include rapid growth in diagnostics market owing to increasing number of diseases and rising healthcare spending. With rising numbers of point-of-care testing (POCT), and advancement in technologies, demand for laboratory medicine and the need for routine biochemical analysis are changing. Increasing R&D for low-cost analyzers is expected to drive the overall market growth.
The advances in clinical chemistry analyzers have enabled labs not just to meet ever-growing workloads, but even to improve turnaround times while achieving consistency in results and minimizing labor requirements.
The India IVD market is likely to exceed USD 1.8 billion mark by 2025, based on the recent survey by ResearchAndMarkets. The IVD industry in India has been witnessing immense progress. Major technological advancements and higher-efficiency systems have taken the sector to new heights. Advanced cutting-edge technologies are being used to understand disease prognosis, thereby strengthening the sophistication level of the participants in the sector.
In India, the emerging trend of corporate players establishing diagnostic centers in small towns and rural areas tends to provide opportunities for the import of automated systems and reagents. One emerging future trend is centered on miniaturized, fully automated, and network-enabled cell phone-based POCT technologies, integrated with paper-based and/or lab-on-a-chip platform. The option of POCT is currently a popular trend in the market since it provides faster results and supports patient-centered approaches to health delivery.
Automation combined with cloud-based technology has helped laboratories streamline daily operations, and better manage patient information. Clinical chemistry has evolved greatly over time, driven by numerous factors, not the least being technological advancements in the world at large. Computers, microprocessors, and robotics have paved the way for automation and cloud-based technology.
BioSystems believes that innovation is the touchstone to keep pace with the rapidly growing technology, and constantly endeavors to offer user-friendly ambience to make diagnostics an affordable yet sophisticated experience.
Industry Speak
![]() AK Vashishth
AK Vashishth
Sr. Vice President,
Beacon Group
Emerging trends in clinical biochemistry testing
Clinical chemistry is one of the several subdisciplines, collectively referred to as laboratory medicine. The practice of clinical biochemistry can be traced throughout the entire history of medicine. The first concepts of urine and blood as a determinant of the disease were published in the 16th Century A.D.
During Middle Ages, physicians incorporated more tests for assessment of patients. The year of 1662 was significant for the discipline of clinical chemistry as world witnessed formation of the Royal Society, and Dr Royal Boyle member of this society, published a paper in 1684, which became a milestone publication in the evolution of chemical examination of blood as an important diagnostic tool.
The novel approaches and discoveries continued and it was until 1923 when the Canadian Surgeon, Sir Fredrick Grant Banting, as the youngest Nobel winner, studied the glucose level in the blood of patients with sugar disease.
From these ancient discoveries till today clinical chemistry has remain as major market.Global IVD Market Valued at US$ 68.12 Billion in 2018 which is growing at CAGR of 5.3%, with major contribution of clinical chemistry. The Indian IVD Market in 2018 is Approximately 1 billion US$ in which contribution of Clinical Chemistry is about 25%.
The market is immensely driven by the increase in the number of chronic lifestyle disorders, cancer incidences, and growing awareness and improving access to healthcare facilities. Today’s laboratories are facing challenges with growing demands by clinicians to provide fast, reliable, and economical methods for testing.
These challenges are largely addressed by using the modern-day technologies like high-speed automation, bio-sensor technology, lab-on-the-chip, and others. Many laboratories are getting equipped with total-automation concept, which utilizes the modular devices that can perform the work of chemistry as well as immunoassay together.
Today, all three important areas like pre-analytical work, analytical work, as well as post-analytical work are getting automated so as to give the best with the least of operator involvement, and getting best accuracy and precision for offering unsurpassed solution toward better patient care.
Second Opinion
 Dr Babli Dhaliwal
Dr Babli Dhaliwal
Director and Technical Head,
Central Lab
A refreshing outlook biochemistry
For three decades and more, the instrumentation and reagents industry has brought about a sea change in the testing of biochemical analytes, be it routine tests or highly specialized parameters. Right from batch analyzers in routine chemistry and immunoassay analyzers to the integrated fully automated systems with a very high throughput, the biochemistry labs have become efficient with very low errors in the analytical phase of laboratory testing.
With innovations taking place rapidly, the manufacturing industry had to innovate better methods of testing and instrumentation and highly stable reagents. Gone are the days of radio immune assays (RIA) using a gamma counter; we now have the CLIA technology with better sensitivity, specificity, and reproducibility. Even the enzyme-linked immune sorbent assays (ELISA) are now available on fully automated platforms, reducing the probability of human errors.
The reagents now have a better stability and a longer expiry period. The need to perform frequent calibrations has also reduced, thereby becoming more cost-effective.
Since most instruments are closed systems, the industry has become more competitive, and even global players had to offer the reagent rental schemes. The advantage of the rental system is that the onus of keeping the instruments in perfect working condition is also shared by the manufacturer. Preventive maintenance and routine maintenance has taken center stage, thereby ensuring the quality of reporting.
The present scenario seems nice but there is a major lacuna in the Indian context. Barring a few, there are no Indian players in reagent or equipment manufacturing companies. There are, however, global companies who make their reagents in facilities located in India. This increases the cost of purchase of instruments and reagents and the fluctuating Indian currency adds to the woes of pricing in terms of cost per reporting test.
The big players in the Indian business industry are even entering the diagnostic field but nobody dares to join the manufacturing industry. Even China manufactures instruments and reagents but India is not even attempting to make the global players to establish facilities to assemble instruments in India, thereby being more cost-effective.
Over more than three decades I have been a witness to so many improvements in biochemistry testing, and the ever-optimistic me hopes that there will be more Indian companies manufacturing all types of instruments in all fields of laboratory testing, leave alone biochemistry.











