Biochemistry Instruments and Reagents
Technology takes center stage

Today there is a new world of automation, one with a complex integration of robotics, liquid handling, and numerous other technologies. These have the fundamental purpose of saving time and improving performance through the elimination of human error and reduced risk of cross-contamination.
Over the last few decades there has been a significant decrease in the number of analytical errors in clinical laboratories, propelled by the fact that laboratories are required to meet very high standards. The technological developments and scientific innovations in the field of clinical chemistry from the early 1950s to date have been vast, enhancing laboratory capabilities and providing the necessary support to clinicians and laboratories to improve patient diagnosis and treatment.
Right from batch analyzers in routine chemistry and immunoassay analyzers to the integrated, fully automated systems with a very high throughput, the biochemistry labs have become efficient with very low errors in the analytical phase of laboratory testing.
Today we are confronted with a new world of automation—a complex integration of robotics, liquid handling, and numerous other technologies with the fundamental purpose of saving time and improving performance through the elimination of human error and reduced risk of cross-contamination.
Now technologically advanced analyzers are capable of automating the process of repetitive sample analysis that was earlier done by lab technicians manually. Manufacturers are developing biochemistry analyzers with multiplexing analyzers. Such type of analyzers possess the feature of positive identification that reduces the process of repeated pathogen testing. This becomes a critical feature in cases of samples that have low volume such as neonatal units. This type of system with shorter turnaround time gives advantages of high clarity and result accuracy. The feature of positive identification helps acquire accurate results in shorter run time by avoiding the inclusion of too many targets.
Furthermore, integrated systems that combine immunochemistry and biochemistry tests are gaining huge attention. It increases the workflow efficiency and delivers fast turnaround and high throughput. It also helps in achieving increased instrument capacity by connecting different analyzer units with a single sample-presentation mechanism. These systems are offered by Roche, Siemens, Abbott, and Beckman Coulter.
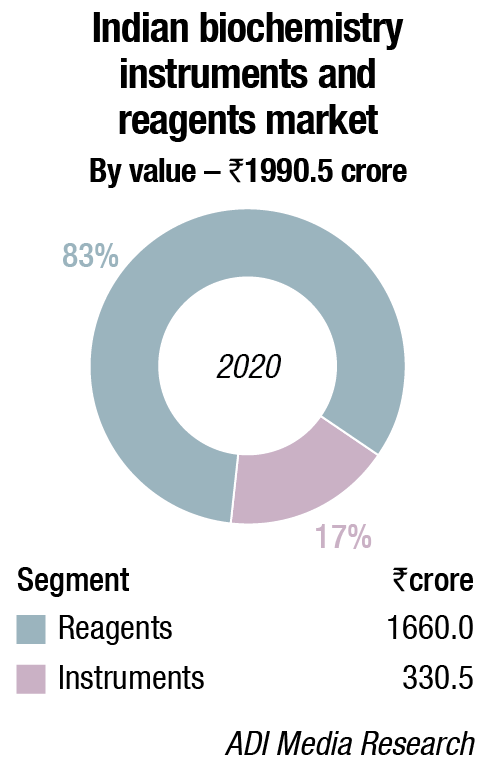
On the other hand, besides pathogen testing, biochemistry analyzers are used for drug monitoring, drug abuse detection, and many more applications. Due to such technological advancements in the field of professional diagnostics, the applications of biochemistry analyzers that were initially restricted to the detection of infectious diseases are now venturing into other areas as well. As a result of this technological evolution, the diagnostics tests are witnessing a boost in their demand.
| Indian biochemistry instruments and reagents market | |||
| Major vendors – 2020* | |||
| FA | SA | Reagents | |
| Tier I | Transasia | Transasia | Transasia, Roche, Beckman Coulter, Siemens, OCD and Abbott |
| Tier II | POCT, Mindray, OCD and Roche |
Mindray, and Robonik | Agappe, Randox, Accurex, Diasys |
| Tier III | Biosystems, Beckman, Abbott, Thermo Fisher, Siemens and CPC | Agappe, Eurit, CPC, Biosystems, Thermo Fisher, Accurex, Tulip, Microlab, Beacon, and regional brands | Biosystems, Fuji, Mindray, Rapid and local brands |
| Others | Trivitron, Sysmex, Tulip, Biosystems, Accurex (Dirui) | ||
| *Vendors are placed in different tiers on the basis of their sales contribution to the overall revenues of the Indian biochemistry instruments and reagents market. | |||
| ADI Media Research | |||
Initially, biochemistry analyzers were used for repetitive analysis that consumed a lot of reagents. This has now changed and due to the replacement by discrete working systems, low volume reagents are now being used. The new instruments are able to automate repetitive sample analysis steps that would have otherwise been done manually by a lab technician. Moreover, as a result of the convergence of system engineering, automation, and IT technology, a significant change has been brought in the global biochemistry analyzers market. The use of ELISAs for clinical testing within a laboratory is time consuming and demands more personnel and resources. However, moving from ELISA technique to an automated biochemistry method increases time and personnel efficiency considerably, and this leads to cost effectiveness as well. Technological advancements are expected to increase even more in the near future, consequently projected to further stoke expansion in the global biochemical analyzer market.
 Recent advancements in clinical chemistry
Recent advancements in clinical chemistry
Thakur Abhishek Singh
Group Product Manager – Biochemistry
Transasia Bio-Medicals Ltd.
“Blood and urine were first analyzed using instrumentation in the early 20th century. Over the years, technological advancements have led to the growth of clinical chemistry to include automated instrumentation to perform hundreds to thousands of tests.
In fact, automation and informatics have shaped the functionalities and design of analyzers:
Technological breakthroughs-Automation has led to increased throughput besides reducing impact of human error and risk of sample cross-contamination. As a result, laboratories are operating with greater efficiency.
Automated analyzers with a small throughput too are integrated with a conveyor for continuous flow analysis with automatic sample loading, mixing, pipetting of sample and reagent. This has greatly reduced the workload of lab technicians and enhanced user- safety by reducing exposure to biohazardous materials. For labs that run round-the-clock, especially during the ongoing pandemic, it allows for faster TAT.
Of late, most analyzers are also equipped with clot detection sensor, prompting the instrument to raise a flag to avoid discrepancies in reporting. Multiple flagging indicates that the sample preparation process needs to be rechecked.
Further, reflex testing is an important function that allows pathologists to add value to reporting. It can assist clinicians with interpretation of results and aid in further disease management for better patient care. Depending on the disease prevalence in a particular area, pathologists can program allied test panels on diagnosing abnormal levels of one analyte. This helps eliminate the need for a repeat sample collection and speed up diagnosis, reporting, and treatment process.
Integrated, sophisticated software, allow ease of calibration management. The software allows programmable scheduling of calibration and reduces the need for intermittent checks.
Artificial Intelligence-Automation with cloud-based technology has helped laboratories streamline daily operations and better manage patient information.
IoT data is used to track usage, expiry, and reagent consumption, for efficient lab inventory management.
Another important aspect, now in the spotlight thanks to the pandemic, is the need for predictive maintenance and 24×7 remote access especially in tier III and IV cities and towns.
Transasia Bio-Medicals Ltd. offers a wide range of fully and semi-automated analyzers with integrated features for smart diagnosis.”
Indian market
In 2020, the Indian biochemistry instruments and reagents market is estimated at ₹3156 crore, with reagents continuing to dominate at ₹1660 crore, at 83.4 percent market share.
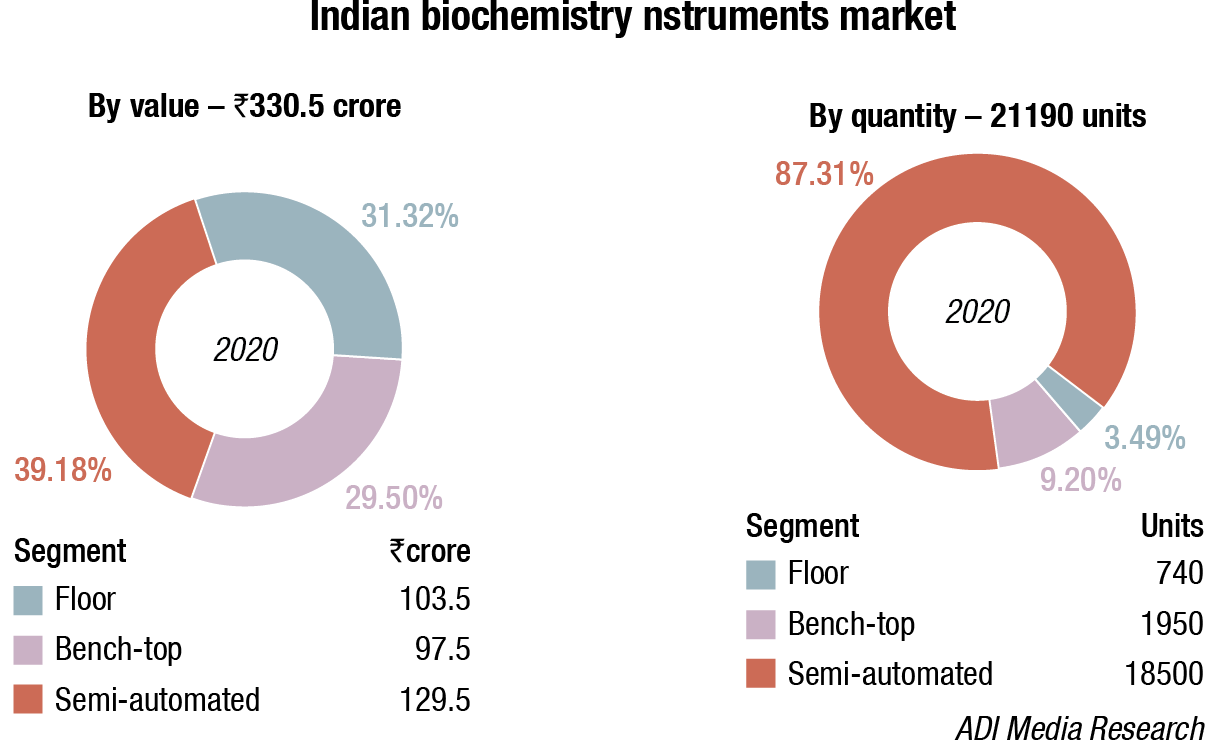
The floor standing analyzers are estimated at ₹103.5 crore and 740 units; benchtop analyzers at ₹97.5 crore and 1950 units; and semi-automated analyzers at ₹129.5 crore and 18500 units. Almost 80-90 percent floor instruments are on rentals, this figure is much smaller for bench-top. Semi-automated instruments are all procured, with almost none on rentals. The size of the market in 2019 has been calculated on assigning a monetary value to all the instruments installed, whether placed or sold.
2020 saw a preference for floor instruments, and this could be explained by the fact that the government procurement share was 71 percent in 2020, out of a ₹201 fully automated instruments market, government procurement amounted to ₹150 crore (detailed table given). These are only for the period February-September 2020, there seem to be no tenders invited after September.
Transasia continues to be a clear leader in this segment, its major contributions coming from the government and the Indian Army. Of the ₹150 crore orders placed by the government, there were some clear winners. POCT secured ₹66.71 crores, GCC ₹10.47 crore, PD Enterprises ₹4.92 crore, SPM Medicare ₹4.44 crore, Metadesign ₹4.36 crore, Chromous Biotech 2.93 crore, Spinco ₹2.5 crore, Transasia ₹2.35 crore, Titan Biotech ₹2.35 crore, Trivitron ₹2.33 crore, Biosense ₹2.23 crore, Avantor ₹2.1 crore, Tulip ₹1.84 crore, P Bhogilal ₹1.59 crore, Bioaid Lab Solutions ₹1.28 crore, PerkinElmer ₹1.28 crore, Rupshee Comm ₹1.17 crore and HiMedia Rs 1.03 crore. Since Agappe represents Mindray, Canon (Toshiba), TokyoBoeki, Furono, and Dirui for fully automated instruments, the company has not been included in the tier table.
 Clinical chemistry – A key segment of lab medicine
Clinical chemistry – A key segment of lab medicine
Rudra Prasad Chakraborty
Product Manager – Biochemistry
Mindray Medical India Pvt. Ltd.
“Clinical chemistry lab is the maximum number of test performing segment in a multi discipline laboratory and is the heart of a tertiary care hospital. From emergency treatment to planned operations everything depends on the clinical chemistry test reports; even treating OPD patients or any clinical condition patient lab reports are indispensable.
Clinical labs not only provide support to physicians’ decision making but also provide key insights in identifying patient condition and illness stage. Lifestyle disorders, cardiac illness, high BP risks are high in Indian population and a lab test can diagnose the condition and risk factor associated with it. Lab Medicine in terms of assessment of inner health picture can guide an individual towards a better prevention pathway from such lifestyle ailments.
In this COVID-19 pandemic also clinical chemistry tests are crucial in patient management and recovery; to name some tests: CRP, D-Dimer, Ferritin are of utmost importance.
To discuss about some of today’s laboratory needs and solutions we land up with the following aspects of a full automated clinical chemistry system. As per todays laboratory needs minimizing reaction volume to minimize patient sample requirement and less reagent consumption is important. This helps specially for pediatric and geriatric patients. Safety features like sample probe clog detection, horizontal and vertical probe movement collision protection, effective interior and exterior probe washing are key to quality result and flawless operation. Optimum number of wavelengths, durable lamp, and maintenance free incubator design to ascertain proper incubator temperature are important for test result integrity.
Also built in bar code reader and bi-directional LIS connection facilities are requirements for today’s needs. The operating software with quality control values monitoring features with automatic L-J graph plotting system is indispensable for maintaining instrument performance records for any accreditation/documentation purpose.
To conclude, in line with our vision and mission we strongly address the current market needs with newer instrument model to provide perfect solution for the middle to high workload laboratories looking for one stop solution for routine chemistries, electrolytes and also COVID parameters, special parameters, like D-Dimer, Ferritin, CRP, HS-CRP, direct enzymatic HbA1c, RA, ASO, IgA, IgG, IgM, IgE, Apo-lipo proteins, micro-albumin and many more on a single work desk.”
Global market
The global biochemistry analyzers market is estimated to account for USD 3716.8 million in terms of value in 2020 and is expected to reach USD 5,429.5 million by the end of 2027, estimates Coherent Market Insights. Increasing number of drug development activities are expected to propel growth of the global biochemistry analyzers market over the next 7 years. For instance, in April 2020, AB Sciex LLC partnered with Intabio, Inc. for accelerating biotherapeutic development and biomanufacturing.
Moreover, increasing prevalence of chronic disorders is also expected to aid in growth of the market. For instance, according to the study, Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition, published in the journal Diabetes Research and Clinical Practice, in September 2019, 578 million people are expected to suffer from diabetes by 2030 and 700 million by 2045.
Fully automated biochemistry analyzers held dominant position in the global biochemistry analyzers market in 2020, accounting for 85.6 percent share in terms of value, followed by semi-automated biochemistry analyzers, respectively.
Rising burden of diseases and steps to reduce them will foster the market growth. Growing initiatives by several government and non-government organizations to control and reduce the burden of diseases will positively impact the industry expansion. For instance, the Population Services International (PSI) incentivizes staff members to identify and mark health conditions with high economic burden. In the last decade, PSI added a goal to target around 42 percent of such diseases and list them under the Global Burden of Disease (GBD) study. This goal will aid organizations to take decisions regarding reduction of disease burden. Such initiatives by the government and non-government organizations will boost the market value over the coming years.
North America market dominates the global biochemistry analyzers market in terms of revenue contribution as compared to that of markets in other regions. This is attributed to the presence of well-developed healthcare infrastructure as well as significant market players in the region. Europe accounts for the second-largest revenue share contribution to the global biochemistry analyzers market, followed by markets in the Asia Pacific, Latin America, and the Middle East & Africa respectively. The market in the Asia Pacific is projected to register comparatively faster growth in terms of revenue over the next 10 years, owing to the developing healthcare infrastructure and increasing funding for research and development by the players operating in the region.
Major players in the market are also focused on launching diagnostic tests for COVID- 9. Major players operating in the global biochemistry analyzers market include Roche Diagnostics GmbH, Siemens AG, Beckman Coulter Inc., Abbott Diagnostics Inc., Mindray, Bio-Medical Electronics Co., Ltd., Hologic, Inc., Randox Laboratories Ltd., Awareness Technology, Inc., Transasia Biomedicals Ltd., and Nova Biomedical Corp.
 Automation in biochemistry – Future ahead
Automation in biochemistry – Future ahead
Shobhit Jain
Product Manager-Biochemistry
Sysmex India Pvt. Ltd.
“Indian in-vitro diagnostic industry is growing at a phenomenal pace with excellent potential to emerge as a global manufacturing hub in medical devices. Especially after success of governments’ Make in India initiative, many multinational companies have started planning to initiate manufacturing in India. After China, Japan, and South Korea, India is the fourth largest market in IVD diagnostics in Asia.
Biochemistry segment continues to dominate the IVD market with largest contribution in the growth of Indian IVD market. The Indian biochemistry market in 2019 is estimated to ₹1920 crore, in which reagents account for 83 percent and Instruments account for 17 percent. High prevalence of chronic diseases, increasing use of point-of-care (POC) diagnostics, rising awareness, acceptance of personalized medicine and companion diagnostics are the major growth factors.
Journey of biochemistry started with very basic measurement systems like colorimeter, flame photometer which was taken over by semi-automatic analyzer and batch analyzers in next decade. Furthermore, with technological advancement (automatic on-board hemolysis for HbA1c, permanent cuvettes, onboard laundry and cooling, clot detection, bidirectional interfacing, Westgard QC reporting systems), integration (modular platform), tools for preanalytical, analytical, and post analytical, the future of biochemistry lies in automation and total lab automation (TLA).
Other socio-economic factors like growing population, increase in health awareness in tier II and tier III cities, increase in medical tourism, resulting in increase in sample load hence there is a growing need of automation which enables fast, cost-efficient, and high-quality testing. In addition to that, labs are also expanding their business by expanding menu, opening new branches, and joining hands with existing market leaders, creating good demand for automation. To conclude, small size to high-end laboratories, private to public sector hospitals, there is a high demand of automation and will be exponentially growing in future.”
Latest trends in automation technology
In today’s healthcare environment, laboratories are responsible for adding value in a way that positively impacts patient outcomes and supports organizational goals through cost-effective care. Central to this is ensuring laboratory efficiency. One of most significant contributors to efficiency over the last several decades has been the development of lab automation. Because automated systems combine multiple functions into a single unit, they also enable laboratories to increase test volumes without added staffing or space requirements. In fact, in some cases, space requirements can be reduced and staffing time freed for higher value work.
The layout of a laboratory has a direct effect on productivity and is foundational to ensuring a seamless, productive workflow. There must be enough space, so work remains unhindered, and employee safety is upheld. Advances in automation have enabled technologists to move away from compartmentalized manual benches, which has given laboratories more opportunities to customize their spaces and design their workflows. Added to this is the availability of a range of systems—from total laboratory automation to pre- and post-analytical modules. This offers laboratories greater control over their workspaces and designs, and allows them to scale products according to test volumes, laboratory size, and cost requirements.
Further, it allows laboratories the flexibility to adapt to and grow with changing needs and opportunities related to technology, patient care, and organizational goals.
Standalone pre-analytical automated systems or benchtop units historically have offered smaller footprints than what is available with total laboratory automation. Thus, they have been cited as a good choice for laboratories with limited floor space.
More and more, vendors are using different variants of machine vision. They are using cameras with the automation to replace the human eye in looking at specimens and looking at the processes to make sure that things are being done correctly. A very basic application is to look at the tube and see if it is the correct tube for the test. In LIS, labs will have the specimen requirement for this test and it may call for a green-topped tube and one can simply look at that tube with a camera and the camera will tell them if it is the tube it is expecting according to their LIS. If it is not, the system can set that tube aside into a special lane where somebody has to now investigate it and correct the error or override it. But depending on people to see if they have got the correct tube for every test, sometime somebody is going to make a mistake. By using the computer and an automation system and cameras, one can greatly reduce human errors and have people looking at problems.
Now it is going to the next level—there are automation systems that some vendors have where they look at the tube and they can see the top of the packed cells and the serum. Based on the diameter of the tube, they can determine the volume of the serum that they have above the packed cells and use that information to guide a pipette tip that’s going to pipette off that serum and put it into a transfer tube, or guide the aspiration tube that’s going to take a portion of the specimen for a test on an analyzer.
One can also use cameras and even more sophisticated technology to determine whether the specimen is hemolyzed, or if it is icteric and it is got the yellowish-green color, or if it’s got excess lipids and cloudiness. In future, the industry is going to see more of this general technology, what is called machine vision, in which cameras are combined with automation to replace the human eye in doing various inspections that are important to quality.
Another area in which automation has significantly improved processes is during pre-analytical activities. These steps include order entry, centrifugation, barcode verification, sample check, and more. This phase of testing is the most laborious and involves a number of steps. Additionally, up to 75 percent of laboratory errors occur during pre-analytical activities. Automating pre-analytical tasks has the potential to make laboratory operations more efficient and safer; however, laboratories must balance the benefits of automation with cost and space constraints.
In today’s healthcare environment, laboratories are responsible for adding value in a way that positively impacts patient outcomes and supports organizational goals through cost-effective care. Central to this is ensuring laboratory efficiency. One of most significant contributors to efficiency over the last several decades has been the development of lab automation.
 RT-LAMP test for COVID-19
RT-LAMP test for COVID-19
Sanjaymon KR
General Manager – Business Development
Agappe Diagnostics Limited
“COVID-19 infected millions of people around the globe. A rapid and accurate diagnosis of the virus is still in need for treatment of the disease and saving lives. Though a bit late, the reverse transcription loop-mediated isothermal amplification (RT-LAMP) based assay came to the market as a great alternative to the commonly used RT-PCR method. This novel process is like conventional PCR tests, with the exception that the nucleic acid amplification occurs at a single temperature, so certain mandatory equipment for PCR, such as costly thermal cycler, is not required any more. This unique nucleic acid amplification method endows the assay to being quicker, easier to use, and more cost effective than RT-PCR assays in the scenario of diagnosis.
RT-LAMP test for COVID-19 has an assay time of 35 min only. The RT-LAMP test was shown to have 100 percent specificity toward COVID-19. Assay sensitivity is also remarkable at 98.7 percent with the limit of detection as low as 4 copies/µL. In this fast and simple-to-use COVID-19 test, 6 LAMP primers target the N gene, and another 6 LAMP primers target the RdRp regions of SARS-CoV-2. The accuracy of the tests is 99.38 percent, and the test results are in good agreement with the conventional RT-qPCR. In detecting viral RNA, the new RT-LAMP assay performs the best, as it can detect positive samples from 10 min of reaction time.
Rapid, low-cost, and user-friendly molecular diagnostic methods are prerequisite to address the outbreaks of infectious diseases. RT-LAMP is an innovative gene amplification technique that shows great promise as a detection tool. One major benefit is the speed of the analysis, as the confirmation of results for the typical RT-LAMP procedure is faster than that of the RT-PCR, by a wide margin. The integration of the amplification and detection step help in making the assay be conducted with faster time to results. Another benefit is the overall ease of use, as the isothermal characteristic of the amplification test ensures that simple and low-cost equipment may be used. The isothermal condition also helps the LAMP to have high amplification efficiency, from the lack of time loss due to thermal cycling in conventional RT-PCR. Due to aforementioned advantages, this assay can be deployed as a screening test in airports and other transit stations, considering the desperate need of increasing the screening capacity for containing COVID-19 pandemic, such RT-LAMP methodology offers a new avenue in molecular diagnosis, filling a role that is vital to track the virus spread.”
Future analyzers forecast
Among many challenges facing manufacturers of analyzers – especially in the era of COVID-19 – one of the top priorities is to remain flexible and ready for anything that comes along. Whether that is from a large spike in coronavirus cases or a treatment-resistant strain of influenza, the diagnostics industry must remain ready to detect and treat whatever possible the next pandemic presents itself in the future.
The analyzer market continues to be as impacted as the rest of the lab industry with the uncertainty and unpredictability that ensues with the current state of affairs. While the extent of the impact brings ambiguity, the need for clinical laboratory solutions to support medical professionals remains constant.
Regardless of what the future holds for the clinical lab analyzers market, the one thing that remains constant is change. And as changes in disease detection and management keep coming, the lab industry and its analyzers will keep meeting the challenges that the new trends present.
 Clinical chemistry tests may help in early stage patient monitoring
Clinical chemistry tests may help in early stage patient monitoring
Swapnil Ramteke
Product Marketing Specialist, Clinical Diagnostics Division, Thermo Fisher Scientific
“The recent studies of the novel coronavirus disease (COVID-19) indicate that most patients tested show elevated levels of C-reactive protein (CRP). In addition to CRP, many patients tested have elevated levels of several other parameters. Among those were alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), ferritin and creatine kinase (CK). Moreover, a d-dimer concentration greater than 1 µg/mL, among other risk factors, could help clinicians to identify patients with poor prognosis at an early stage.
IFCC recommends testing CRP levels from COVID-19 patients among several other common IVD parameters – IFCC Information Guide on COVID-19.
Elevated levels of CRP may indicate severe viral infection. C-reactive protein (CRP) is a normal constituent of serum present in healthy individuals in very low concentrations. Invasive bacterial infection and extensive tissue damage cause increased CRP levels. Levels in plasma usually rise dramatically after, e.g., myocardial infarction, trauma, infection, inflammation or surgery. The increase begins within 6 to 12 hours, and level may reach 2000 times normal. Determination of CRP is clinically useful for screening for organic disease, assessment of the activity of inflammatory disease, detection of intercurrent infections or after surgery. According to IFCC, elevated levels of CRP may indicate severe viral infection, viremia, or viral sepsis in COVID-19 diagnosed adult patients.
Monitoring iron therapy. IFCC recommends testing Ferritin levels from COVID-19 patients among several other common IVD parameters.
An aid for the diagnosis of venous thromboembolism and coagulation. IFCC recommends testing D-dimer levels from COVID-19 patients among several other common IVD parameters. Elevated levels of D-dimer may indicate activation of coagulation and/or disseminated coagulopathy in COVID-19 diagnosed adult patients. Increased concentrations of D-dimer may include venous thromboembolism (VTE), arterial thrombosis (including myocardial infarction and stroke), disseminated intravascular coagulation (DIC) association with recurrent thrombotic risk following anticoagulation, post-operative state, significant liver disease, malignancy and normal pregnancy. D-Dimer testing has become a useful laboratory tool for the diagnosis of VTE because it has high negative predictive value when used in combination with pretest clinical probability.
Choosing right clinical chemistry analyzer in the time of pandemic. Across the globe general safety and occupational safety of healthcare workers has become the priority. Authorities are taking all possible measures to reduce the community spread. Hospitals and COVID centers are focusing on waste minimization and proper waste disposal. From the biochemistry laboratory perspective, liquid waste generation, and waste disposal is a huge concern in the COVID times. Hence it is highly recommended to place analyzers with disposable cuvettes so that there is zero carry over of samples and one must look for an analyzer where there are no external tubing or cans for waste disposal. Also, the water consumption should be least, as more water being used, more waste would be generated. WHO is preferring such analyzers for their COVID centers.”











