COVID-19
The answer lies in ubiquitous testing!
Economic and social activity cannot be kept at bay anymore. COVID needs to be accepted as part of our lives.
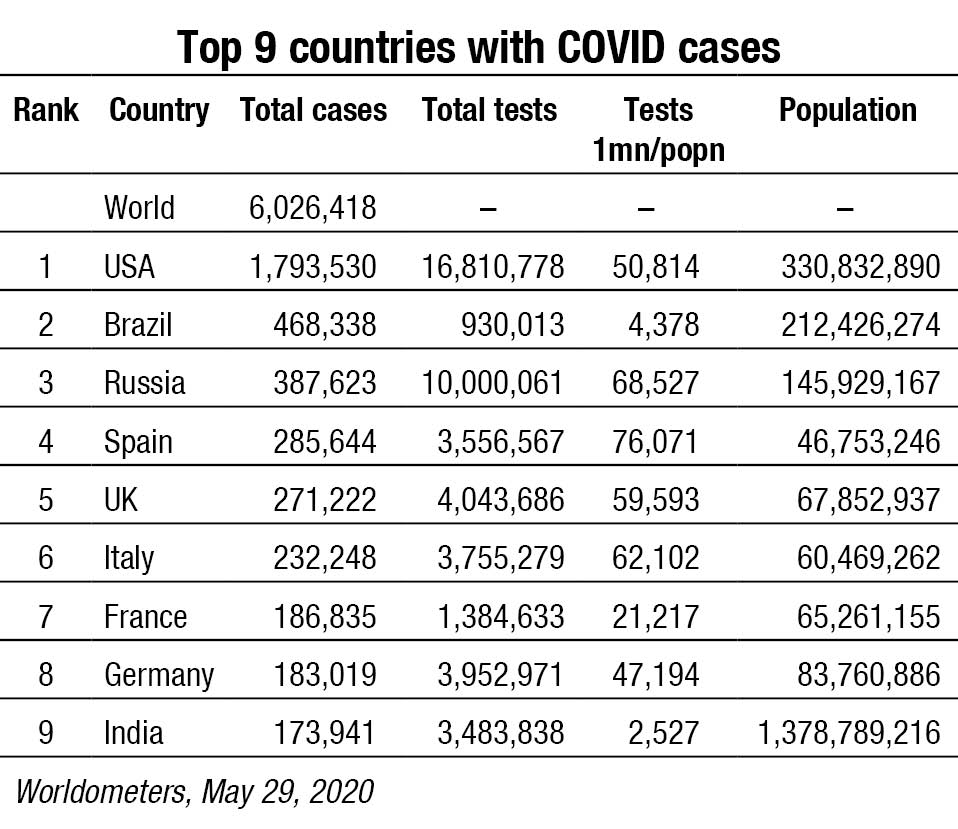
As on May 31, India had held 2710 tests per one million population. It has gradually moved upto rank 7 in terms of total COVID cases. This is an absolutely pathetic number compared to Spain’s 86,921 or USA’s 53,417 tests per million population. China, which has a similar population of 1.43 billion as compared to India’s 1.37 billion has not declared its figures. At this rate, close to two years would elapse before 5 percent of Indians are tested once.
Given the massive size of our population, the coronavirus can only be contained, once the number of tests increase manifold, that is, until a vaccine is developed. The virus currently has no effective treatment, and a safe vaccine is possibly a year away. Moreover, even recovery from infection does not guarantee immunity from future infection.
The number of tests would have to go up from 150,000 daily to one million. And it is not an absurd expectation. The US is aiming for a million daily tests. Germany already has walk-in test centers.
The government must let go of restricting that tests can only be done in approved labs. Tests must be done by all certified labs as the regular blood tests are done today. India has a wide network of labs in the country. Collection centers must be set up in every village, in every neighborhood. This would be very similar to the H1N1 samples collected from the doorstep. En masse testing is the only way to know the varieties of the virus or its mutations.
As the economy opens up, offices will want their employees tested before they resume work, as will the airlines before the passengers board a flight. Similar requirement will come from construction sites, hospitals, banks, and other public places. The country needs to start living again, shopping, dining out, vacations, and more. These actions sustain a whole ecosystem of businesses and livelihoods.
Funding these tests will be a major issue, that is till the costs come down. The corporate sector, NGOs, and other innovative methods will need to be found to share the burden with the government.
Shortage of swabs, reagents, and POC testing machines will need to be addressed. Reagents continue to be imported from Europe, the US, and China. Indian companies do not make them. The global companies make them at scale, and offer optimal pricing. Stockpiling of reagents will begin now as the whole world is looking for reagents.
India will need to become self-reliant in producing diagnostics tests, and to bring the cost of testing within an affordable range. Currently, manufacturing kits locally is not an option for most manufacturers. There is little to no access to antigens and other chemicals required to do so. The Department of Biotechnology and its public sector undertakings, Biotechnology Industry Research Assistance Council; NITI Aayog’s new initiative Project CARD; Gujarat Biotechnology Research Centre, in partnership with Neuberg Supratech Reference Laboratories (NSRL), and many other efforts are underway. Chennai-based Trivitron Healthcare and Pune’s Mylab Discovery Solutions are awaiting regulatory approvals for their kits.
ICMR and NIV will have to change their stance. They have both become regulatory behemoths, with no transparency in their operations. Their insistence on 100 percent concordance is against international norms. No rapid antibody kit in the world shows 100 percent sensitivity or specificity. Even a textbook definition talks of anything between 96 percent to 98 percent. Manufacturers are opting for FDA and EU approvals, as then they can go to market immediately. All they need is permission from the DCGI to import. Also, competent institutes as National Centre of Disease Control, Christian Medical College at Vellore, and Sanjay Gandhi Post Graduate Institute of Medical Sciences at Lucknow have been completely sidelined.
Rapid testing could hold the key to testing millions. Most of these tests come from China, and can potentially be self-administered, like pregnancy test kits. Potentially, rapid tests, which are often pin-prick, point-of-care devices, provide the strategic advantage of screening people en masse. They provide a scientific way to quarantine people instead of blanket lockdowns.
A certificate in the form of a pass, à la Aadhar card of COVID testing will need to be given to each and every citizen and the economy brought back to life. Many issues need to be addressed, but the government must first come out of the lockdown mode into the functioning mode. The country cannot afford the luxury of a comatose economy.












