MB Stories
The COVID-19 pandemic seems to be heading to become endemic

The third wave of COVID-19 in India is a near certainty, which is the issue we need to address in the near future. And then the issue that the virus and its variants are here to stay. The virus will probably keep mutating and circulating amongst the people. Alongside this, the pharma companies will likely continue to create vaccines, until the virus mutates to becoming less deadly, and over time, becomes seasonal like influenza.
2021 will be a year not easily forgotten. India has found a place in the world map for all the wrong reasons. The dreaded coronavirus seems to be running its course in the entire 1.4 billion population. And mutating into new variants, the latest being B.1617.
An entry driven by the B.1.617 spike protein is partially resistant against neutralization by antibodies elicited upon infection or vaccination with the Pfizer-BioNTech shot, the main lines of defense in developed countries.
The world is worried. Other countries have begun to open up. London is getting back to dining out again and the US is celebrating mask-free shopping. Yet one-fifth of its population is facing fresh restrictions. Thailand has extended its ban on travellers from India. While travel ban from India to the US impacts non-immigrants and non-citizens of the United States that have been in India for any or all of the last 14 days, Australia refused entry for even its own citizens coming home from India. The Group of Seven meeting in London, where India’s foreign minister and his entire team had to self-isolate after two delegation members tested positive is one stark instance where India justifies being ostracized.
More recently, Singapore has had to regress as the B.1.617 variant, has been found circulating locally. And it is only till when the entire country can be vaccinated, by the end of 2021. The situation in India offers a stark contrast to this.
COVID-19 has left the citizens of India completely defenseless, vulnerable and totally broken. None have been spared, neither young nor old, rich nor poor, urban nor rural. Those alive struggle to find hospital beds, oxygen cylinders or life-saving drugs, while the unfortunate ones are seeking firewood and a place to cremate their loved ones. Scared villagers are dumping bodies into the river.
There has been huge anger against the present government at total disregard displayed for lack of vision, planning, or empathy. Prime Minister Narendra Modi’s administration could have done many things differently to prevent overwhelming the country’s patchy health system.
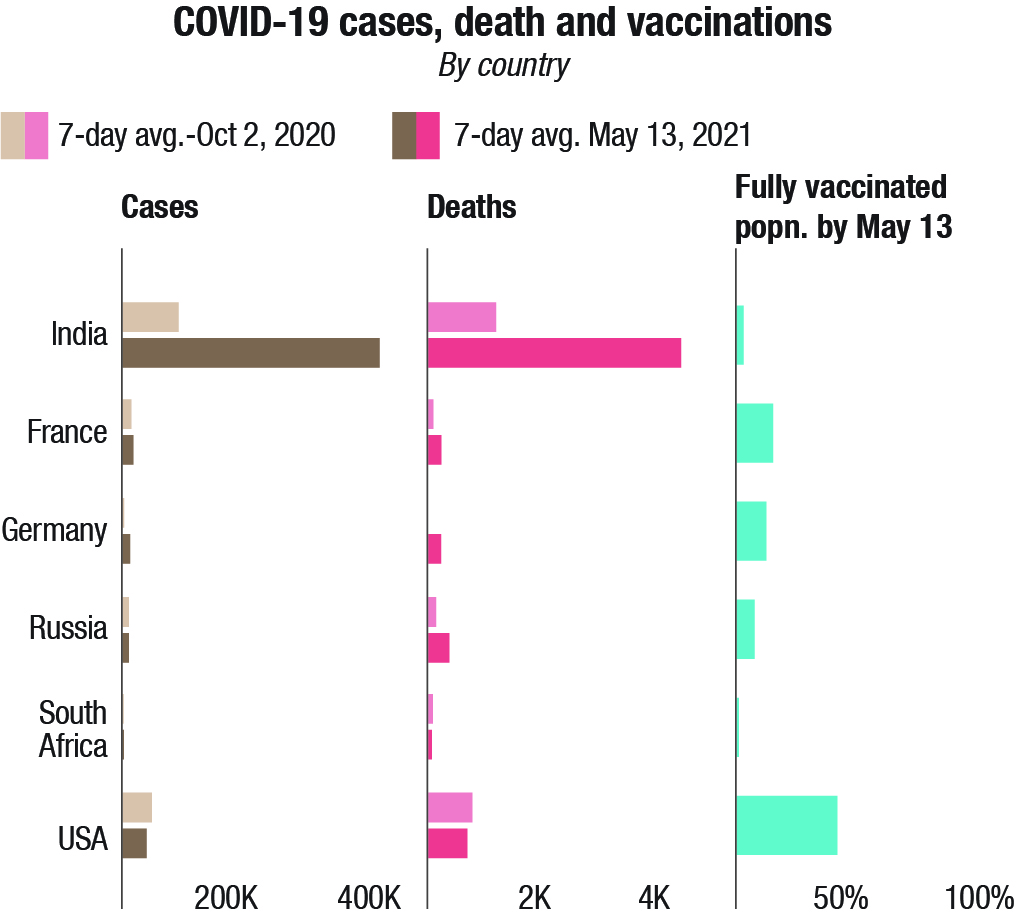
One is beyond guessing the true extent of the unfolding disaster in India. Whether daily fatalities and new cases are, as officially reported, around 4,000 and 400,000, respectively, or closer to 25,000 deaths and between 2 million and 5 million infections, as Brown University School of Public Health’s Ashish Jha estimates.
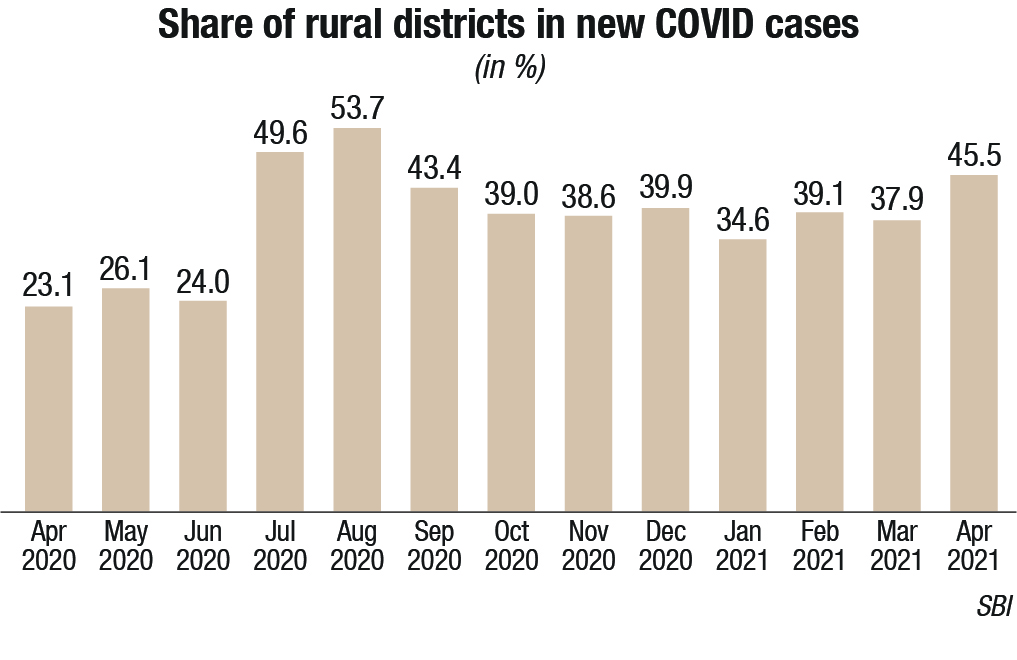
India has slid into the world’s worst health crisis. One research model is predicting, deaths could rise to 10.19 lakh by end July from the current official count 3.07 lakhs of May 25. Just as some countries needed ventilators in large quantities last year, India is now desperately seeking oxygen supplies and concentrators. And the next big worry is that the pandemic is clearly seen moving to semi-urban and rural areas. And forget oxygen supplies, most villages do not even have healthcare facilities or doctors. Entire families are dying from the disease. And unlike India’s social-media literate urban population, villagers cannot appeal on Twitter for help.
As infections increased, the pace of vaccinations was not fast enough to mitigate the second wave. India has administered 198.5 million doses by May 24. However, the sheer size of India’s population means only 11 percent of Indians have received at least one dose, and fewer than 3.3 percent are fully vaccinated.
India’s current domestic vaccine production capacity of 70 to 80 million doses per month will not be enough to meet its goal of fully vaccinating 300 million people by July, never mind its contractual commitments to COVAX, the international effort intended to provide equitable vaccine access to the world’s poorest countries.
India has an adult population of about 1 billion people, and most vaccines that will be available in the country require two doses for full vaccination. With some wastage factored in, the 216 crore doses – 2.16 billion – should be enough to cover India’s needs. Domestic vaccine makers remain unconvinced about the government’s claim that India will have the requisite 2.16 billion doses by the year-end.
The health ministry’s assumptions on raising vaccine production indicates how far removed the government is from ground reality, given continuing uncertainties about raw material supplies and approvals and the fate of upcoming vaccine candidates winding through clinical trials.

As per government projections, about 550 million doses of Covaxin are expected to be produced by December 2021 by Bharat Biotech and three public sector units, Indian Immunologicals, Bharat Immunologicals and Biologicals, and Haffkine Biopharmaceutical Corp. through a technology transfer. All three companies either have to repurpose or set up new plants. Bharat Biotech has also enhanced manufacturing capacities at Chiron Behring Vaccines, Ankleshwar Gujarat, a wholly-owned subsidiary. The product availability at Ankleshwar is to commence from the fourth quarter of 2021. Bharat Biotech had already deployed multiple production lines at its Hyderabad and Bengaluru campuses. Covaxin production, currently at 10 million doses a month, will be scaled up to 60-70 million by August, 100 million by September, and finally to one billion doses per annum.
| Vaccines | Doses (cr.) | Status |
|---|---|---|
| Covishield | 75 | 11 cr doses in May-July |
| Covaxin | 55 | 5 cr doses in May-July |
| Biological E | 30 | In phase 3 trials |
| Zydus Cadila | 5 | In last stage of phase 3 |
| SII-Novavax | 20 | Approval pending |
| BB Nasal | 10 | In phase 1-2 clinical trials |
| Gennova | 6 | In phase 1-2 clinical trials |
| Sputnik | 15.6 | Available |
| Total | 215.6 | |
| Additionally, other foreign vaccine may also become available. | ||
| Vaccine | Developer/Manufacturer | Status |
|---|---|---|
| ZyCoV-D (second indigenously developed vaccine) | Zydus Cadila | Supply is likely to begin in June 2021 |
| Covovax (SSI’s version of NVXCoV2373) | Developed by Novavax (USA) and manufactured by SSI |
The companies entered into an agreement in August 2020 and SSI is expected to test the safety of Covovax. The institute intends to begin stockpiling in April and is targeting production to reach upwards of 40 to 50 million doses per month. |
| Biological E Limited vaccine |
Biological E. | The company is set to launch Phase III clinical trials after being granted approval from the CDSCO Subject Expert Committee |
| HCG019 | Gennova Biopharmaceuticals | The Department of Biotechnology in Ministry of Science & Technology announced in April 2021 that it has approved additional funding toward clinical studies of India’s “first of its kind” mRNA-based COVID-19 vaccine. |
| BBV154 | Bharat Biotech | The intranasal vaccine is currently undergoing Phase I trials in the cities of Patna, Chennai, Hyderabad, and Nagpur. |
| UB-612 | Aurobindo Pharma in an agreement with COVAXX (USA) |
The collaboration expects to submit clinical trial data in July 2021 and expects approval within the financial year |
| Carnegie India |
About 750 million doses of Covishield are projected by the government by December 2021. Covishield maker Serum Institute, currently makes 60-70 million doses a month, and plans go up to 100 million, once its capacity expansion is completed in July. The company expects 500 million doses in the remaining five months of the year.
Sputnik V, from Dr Reddy’s Laboratories expects to give 20-25 million doses every month, once its production is scaled up, amounting to a maximum of 125 million doses by year end. The challenge of the second doses being different from the first one, and needing to be separately manufactured, is not being taken into consideration.
In the fray are 700 million doses of various experimental vaccines. The government’s projections have included vaccine developed by Novavax, being made in India by the Serum Institute of India under licence, but not yet been approved anywhere in the world; a nasal vaccine from Bharat Biotech; an mRNA vaccine from US-based HDT Bio Corporation, in partnership with Gennova Biopharmaceuticals, that have barely begun early trials; and Zydus Cadila’s ZyCoV-D. Biological E., Hyderabad, also plans to produce 300 million doses of the vaccine developed by scientists at the Texas Medical Centre in the US and California-based Dynavax Technologies, for which phase 3 trials have only just begun in 15 locations across India. Biological E. is looking to contract-manufacture about 600 million doses of the J&J vaccine annually too.
No doubt, although the numbers will likely be relatively small, some vaccines may find their way into India from other countries, from USA for example. Now that the global vaccine manufacturers have refused to entertain the States, and will deal only with the Union Government, in all probability the Centre will import and distribute vaccines.
As the majority of the country remains unvaccinated, the vaccine manufacturers may scale up production, but only to keep producing boosters, and as new variants emerge, vaccines for those will be needed too.
Then there is the issue of efficacy. Some vaccines are better than others. Evidence derived from the expanding global inoculation rollout indicates that the messenger RNA shots developed by Moderna Inc., Pfizer Inc. and BioNTech SE are better at reducing onward transmission and preventing asymptomatic infections. In countries such as Israel, Qatar and Malta new COVID cases are declining as vaccinations spread. These more traditional vaccine types have shown efficacy rates of between 50-80 percent in preventing symptomatic COVID in clinical trials, compared with more than 90 percent for mRNA ones. As the mRNA shots require ultra-cold storage, it limits their accessibility to countries with poor transport and storage infrastructure. Different transmission rates of virus variants also play a part. Of course, people who received shots, mRNA included, still get COVID.
In comparison, in India, the first dose of Covaxin does not achieve much of antibodies, these are achieved after the second dose. With Covishield, antibodies are achieved at good levels. This is seconded by Balram Bhargava, Director General of Indian Council of Medical Research (ICMR).
It would be a folly, or rather suicidal not having learnt a lesson from the second wave, when we were caught napping, or rather celebrating that India had successfully controlled coronavirus. It would be prudent to prepare and strengthen the healthcare system for the next wave. The writing is clear on the wall. As the virus mutates further, the third wave of COVID-19 in India is a near certainty.
The country had gone through a nightmare, in the eight weeks between March 1 and April 30, 2021. India’s daily cases grew 40 times from 11,000 to more than 400,000. No healthcare system in the world can handle an explosion of patients of this sort. The only way to protect the population is to stockpile equipment that may not be immediately required today but are produced and procured and set aside in anticipation of a wave’s peak.
However, amid this doom and gloom, if the government is even now able to commit itself to course-correction, the situation can somewhat be saved. If strong measures are taken and effectively implemented at the state, district and city-level, the third wave may not happen in all places or indeed anywhere at all.
National Association of Software and Services Companies (Nasscom), comprising large Indian companies including software development, software services, IT-enabled and BPO services are seeing double-digit growth rates as clients increasingly invest in technology to transform their businesses. Their president, Debjani Ghosh has urged the government to allow procurement of vaccines approved by the World Health Organisation (WHO) and liberalise the Foreign Contribution Regulation Act (FCRA) to ensure that its customers and individuals can provide relief and contributions to tackle the pandemic. “For India, with a population of 1.3 billion, the only way is to have public-private partnership in procuring, distributing vaccines, and ensuring that every person is vaccinated,” said Ghosh.
The coronavirus seems to be here to stay. The virus will probably keep mutating and circulating amongst the population. The CEOs of Pfizer and Johnson & Johnson, both have said that there may be a need to get vaccinated annually against the virus. Dr. Anthony Fauci, Director, National Institute of Allergy and Infectious Diseases, USA supports this, “We know that the vaccine durability of the efficacy lasts at least six months, and likely considerably more, but I think we will almost certainly require a booster sometime within a year or so after getting the primary.”
A recent sero-survey by the Institute of Genomics and Integrative Biology (IGIB) suggested that the neutralising antibodies declined significantly after five-six months, making people prone to reinfections.
Alongside this, it is very likely that newer and newer variants will keep emerging, until the virus mutates to becoming less and less deadly over time, and it gets concentrated in local, smaller populations or even become seasonal, like the flu. How many more rare, life-threatening COVID-19 complications as mucormycosis rear their ugly head now is anybody’s guess.
The pandemic, although far from over, will likely become endemic.











