Coagulation Instruments and Reagents
Three brands continue to dominate the Indian coagulation market
The coagulation segment saw traction in D-dimer testing as SARS-CoV-2 induces intense D-dimer elevation, and monitoring improves prognosis.
As India gears up to vaccinate its 1.3 billion citizens, it is important to understand the pathophysiology and clinical manifestation of the infection for preventive and treatment strategies. The laboratory has a clear role in diagnosis of COVID-19, and monitoring viral shedding; it remains less clear at the present time how other laboratory testing can contribute.
This outbreak of COVID has positively affected the Indian coagulation testing market and is anticipated to witness a noticeable growth during this outbreak. This expected growth is attributed to recent studies that suggest that respiratory problem in COVID-19 patients is not the only reason for the development of acute respiratory distress syndrome in patients, and this respiratory issue also happens because of blood coagulation. Results from recent studies suggest measurement of D-dimer and procalcitonin concentrations are helpful in the early assessment of COVID-19 patients with comorbidities like coronary heart disease, coagulopathy, and co-infection, who require hospitalization.
COVID-19 has created a shift in the market segment, and there is a huge demand for coagulation-associated parameters like D-dimer. The increase in D-dimer was the most significant change in coagulation parameters in COVID-19 patients, and occurred more frequently than other coagulation parameters, such as prothrombin time (PT) or aPTT. The recently published IFCC guidelines on COVID-19 strongly suggest D-dimer testing in patients with COVID-19 since studies on SARS-CoV-2 revealed a high correlation between severity and outcome of COVID-19 in patients with increased D-dimer levels. Physicians are preferring D-dimer tests as potential prognostic marker to assess the disease severity of the COVID-19 patients. Also, serious novel COVID-19 pneumonia patients have thrombosis (blood clot) and this requires continuous monitoring of patients during treatment. This aspect is eventually fueling the demand for coagulation testing procedures, which is expected to accelerate the market growth in the COVID-19 outbreak.
With an increase in the number of COVID-19 cases in India, manufacturers are witnessing a rise in demand for COVID-19 parameters like D-dimer, Ferritin, and CRP, and also coagulation factor VIII.
Amid the COVID-19 crisis, the global market for D-dimer testing, estimated at USD 2.1 billion in the year 2020, is projected to reach a revised size of USD 2.7 billion by 2027, growing at a CAGR of 3.8 percent. Point-of-care (POC) tests segment is projected to record 4 percent CAGR and reach USD 1.1 billion by the end of 2027. After an early analysis of the business implications of the pandemic and its induced economic crisis, growth in the laboratory tests segment is readjusted to a revised 3.7 percent CAGR for the next seven-year period. The significance of monitoring D-dimer levels in COVID-19 cases during treatment, along with the augmented demand for the techniques of advanced coagulation testing, is estimated to pave way for rapid growth of the global D-dimer testing market in the near future.
The global coagulation testing instruments and reagents market is expected to reach a value of USD 3814.3 million by 2026 at a 7.1-percent CAGR from 2020 to 2026.
By product type, consumables held the global market share in the previous years and are estimated to account for USD 1975.8 million by 2026. Consumables contain strains and reagents; all testing procedures require reagents, thereby wide consumption and repeated orders of consumables for coagulation testing. This factor is projected to drive the market size. Instruments segment will grow at a healthy rate of 7.4-percent CAGR, and is estimated to reach up to USD 1838.5 million by 2026. Growing diagnosis centers and developments in diagnostic analyzers, along with enhanced monitoring procedures, are projected to drive demand for instruments.
The market for prothrombin time application accounted for the highest coagulation testing market size in 2020, and is projected to generate revenue of USD 2648.1 million till 2026. This growth is attributed to wide usage of coagulation testing procedures for prothrombin time application in hospitals to evaluate the hemostatic system. Besides prothrombin time, the market for APTT accounted for second-highest market share in the global market, and is expected to register for USD 639.2 million in 2026.
By end-users, hospital end-use accounted for majority of the coagulation testing market share in 2020, which was about 37 percent, and is projected to continue its growth over the next five years. Increasing number of patients admitting in hospitals due to growing prevalence of chronic disorders requires continuous monitoring; this factor is projected to enhance the market size in coming years. POC testing segment will grow at a significant CAGR of 8 percent, and is projected to create lucrative opportunities in the global market. This expected growth is attributed to government support, along with the increasing number of POC testing centers for diagnosis.
Europe region has dominated the global coagulation testing market size in 2020, and is projected to account for USD 1171.0 million by 2026. Presence of well-developed healthcare infrastructure and increasing geriatric population, coupled with rise in occurrence of chronic diseases, are projected to drive the market growth in the European region. Asia-Pacific market will grow at a notable CAGR of 7.5 percent, and is estimated to reach up to revenue of USD 907.8 million by 2026. Increasing awareness on early diagnosis and government support for development of healthcare infrastructure are the favorable factors for the growth of market in Asia-Pacific region.
Over the past decade, rapid advancements in technology and the introduction of new coagulation analyzer tests have led to an increase in the quality and efficiency of hemostasis laboratories. Some of the modern complex coagulators also possess high throughput, flexibility, and reliability. Other than this, they provide improved accuracy and precision, and easy-to-use advanced software, provided with in-built graphs and calibration curves. Moreover, there has been a significant rise in the prevalence of cardiac diseases and blood disorders, which has created the need for improved coagulation analyzers across the globe. Besides this, the sales of coagulation analyzers are positively being influenced by the increasing number of hospitals, diagnostic centers, and research institutes established worldwide.
The major global players operating in the coagulation analyzers market are Abbott Laboratories, Danaher, Siemens Healthineers Group, Sysmex Group, Thermo Fisher Scientific, Horiba, Nihon Kohden, Roche Diagnostics, Maccura Biotechnology Co. Ltd., Bio Group Medical System, Genrui Biotech Inc., A&T Corporation, Beijing Succeeder Technology Inc., Diagnostica Stago SAS, Helena Laboratories, Hycel, Eurolyser, Bpc Biosed Srl, ERBA Diagnostics, Inc., Sclavo Diagnostics International S.r.l., Haematonics, Biosystems S.A., and ACON.
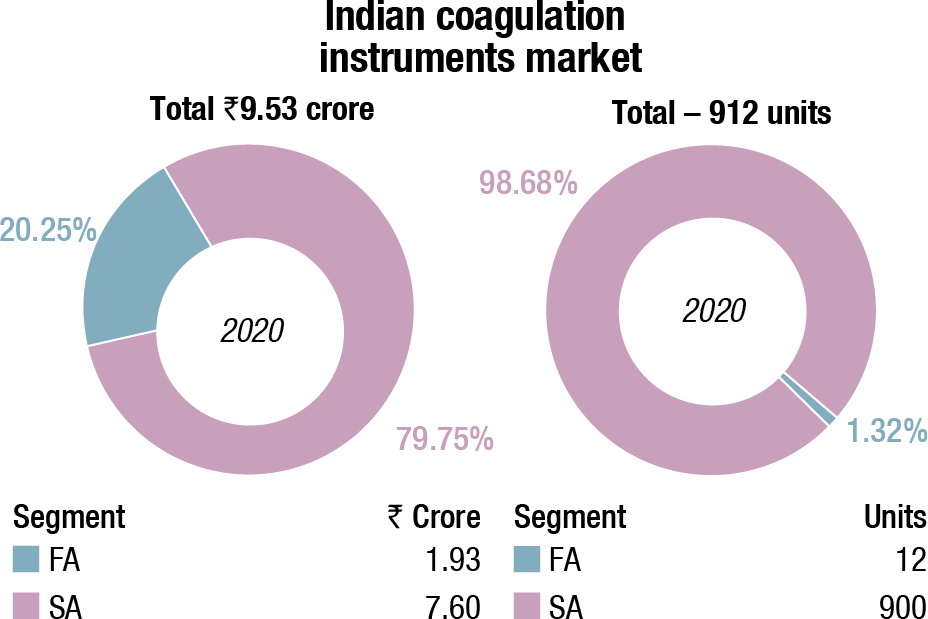
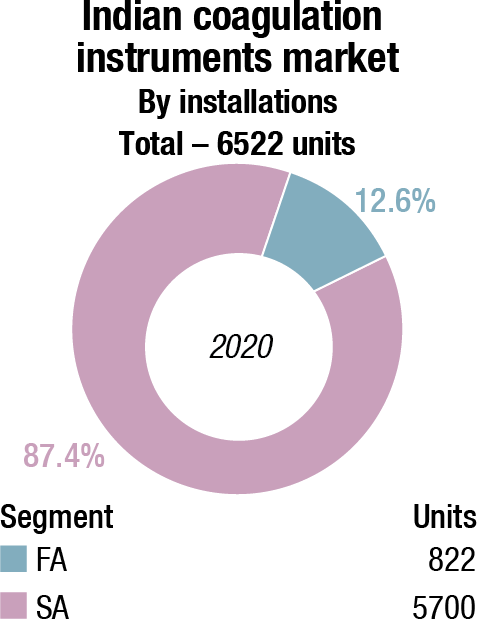
The Indian coagulation instruments and reagents market in 2020 is estimated at ₹153.53 crore. The value of the fully automated instruments that were sold is ₹1.93 crore, with 12 units being sold, and semi-automated as 900 units, estimated at value ₹7.6 crore. An additional 120 fully automated and 100 units of semi-automated instruments were placed. The three leading players remain Stago, Werfen, and Sysmex.
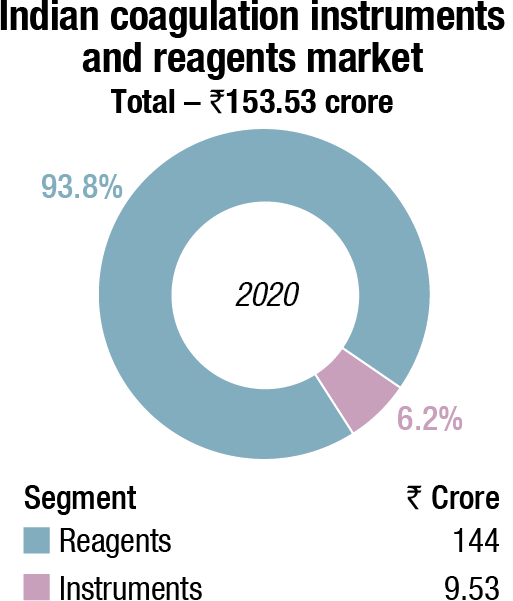
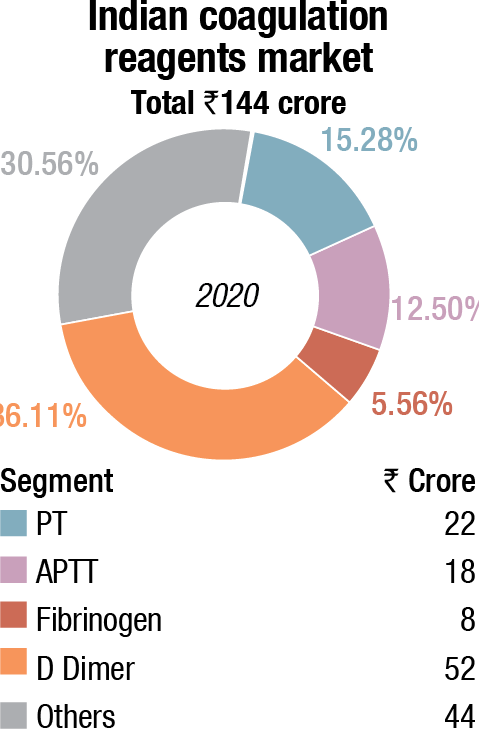
The coagulation reagents took a major dip in 2020. The D-dimer tests were the savior of the coagulation market. Once COVID-19 peters out, demand for D-dimer test shall peter out, yet the market size is expected to have stabilized at double the market from the pre-COVID days. While satellite centers of laboratory chains had D-dimer analyzers placed, the small laboratories used semi-automated instruments to conduct the tests.
| Indian coagulation instruments market | |||
| By vendors – 2020 | |||
| Tier I | Tier II | Tier III | Others |
| Stago (including Trinity) | Werfen India & Sysmex | Tulip, and Transasia | Agappe, Trivitron, Compact Diagnostics, CPC Diagnostics, HUMAN, Meril Diagnostics, and Roche |
| *Vendors are placed in different tiers on the basis of their sales contribution to the overall revenues of the Indian coagulation instruments and reagents market. | |||
| ADI Media Research | |||
As a trend, the three brands reported that while they were not able to sell a large number of fully automated instruments, average unit prices not only declined but there was a huge variation between the final prices. Additionally, the manufacturers’ exposure increased in 2020 as more analyzers compared to 2019 and 2018 were placed, and that is expected to give better return over the next few years. There is a huge expectation that the reagents market in 2021 shall increase, before it settles down at a stable, normal level by 2022.
ML and coagulation testing. Automation has prepotently revolutionized workflow and sample management in modern laboratories, most of which takes profit from the concept of lean manufacturing, which entails eliminating or minimizing all steps and activities that do not add value to the whole process. Several technical solutions exist that can be customized around specific needs of organization and testing volume. Coagulation testing has remained virtually apart from this innovation for a long time, and only recently has the concept that coagulation analyzers may be combined within a model of (total) laboratory automation started to emerge. Some recent studies have shown that automation solutions and connection of coagulation analyzers to track-line systems are reliable strategies for improving efficiency (i.e., throughput and turnaround time) and decreasing costs in clinical laboratories, with negligible effects on the quality of routine hemostasis testing.
Recent years have seen the advancement of the use of robotics in the laboratory setting. The use of automation allows for many processes that once required a considerable amount of resources dedicated to them, such as time and human workers, to be conducted at faster speeds, with greater levels of consistency, permitting the conservation of time and human resources within the laboratory.
Coagulation testing is one such procedure that laboratory robotics has automated. Previously, it has been explored whether the process of analyzing hemostatic samples could reliably be automated.
The key concern was the vulnerable nature of the samples; they are prone to detrimental impacts via the movements that automation introduces to samples, such as track, belt, or conveyor transportation.
Early studies demonstrated that robotic handling might potentially affect the quality of the coagulation testing results. Samples for hemostatic testing are centrifuged before analysis. Automated transportation systems of these centrifuged samples may cause partial resuspension of pellet cells within the upper plasma phase. This has been of concern when the samples were transported at high speeds. Previously, automated systems have been compared to manual direct loading into the analyzer. Although significant differences were found between the samples that were directly loaded into the analyzer, compared to samples that were entered via the automated track-line system, the results of the track-line system were still within the total allowable error.
It has been demonstrated that the processing of plasma samples through extensive track-line systems affects the results of routine hemostasis testing. Nevertheless, as the differences were not focused clinically significant, the automated processing of hemostasis testing remains a viable option.
Coagulation segments of IVD in India
 Thomas John
Thomas John
Managing Director,
Agappe Diagnostics
In coagulation market scenario, India has shown tremendous growth in the market, especially after the pandemic. D-dimer as a COVID prognosis parameter has boosted the single market multi-fold and the trend continues to be unabated still. Otherwise, PT, APTT, fibrinogen testing, platelet function testing. Coagulation analyzer market size should have 5.5 percent CAGR, at par with universal trend during 2021–2027. Indian coagulation market is predominated with semi-automatic analyzer systems, due to the peculiar market response, apart from very large multi-speciality hospitals.
The pandemic has boosted the hardware business in this sector due to extensive utility of the coagulation system, a post COVID scenario, where clinicians want to have instant reports for emergencies. POC systems also gain momentum these days. Growing chronic blood disease cases will significantly boost the coagulation testing market in coming years. Coagulation market in India in 2020 is estimated to be ₹200 crore, with 25 percent contribution from equipment side and 75 percent from reagent side.
Agappe, your best partner in diagnostics, is the biggest supplier of D-dimer in India during the COVID pandemic; we had sold around 10 million tests of D-dimer during the COVID pandemic to serve the nation in pandemic for the COVID prognosis, when our country faces acute shortage for the product at times. At present, Agappe provides semi-auto analyzer systems (Mispa Clog) for analyzing coagulation parameters. Further, we plan to come up with new automated platforms with multiple parameters to cater to the growing demand in India.
The impact of movement on the samples must be considered to limit the negative effects on the quality of the results.
Indeed, the automation and high throughput means that extra care needs to be placed on ensuring sample integrity. Thus, it is not good enough to get a fast PT or APTT (for example), one need to ensure that these test results are accurate and reflect the patient’s status, and not just the sample status. Modern instruments are generally very reliable; nonetheless, errors in test results can occur. Importantly, pre-analytical issues account for the majority of test errors in modern times. Such pre-analytical issues often arise due to poor blood collection, and the arising sample activation and clotting prior to receipt by the laboratory. The major outcome is the development of hemolysis and also partial or complete clots. Neither is easily identified by visual inspection of the whole blood, unless the clot is large enough to prevent blood mixing; however, both can certainly compromise the sample quality. Typically, samples arrive in the laboratory, patient details and ordered tests are entered into the laboratory information system (LIS), and the collection tubes are quickly centrifuged. The primary sample, with the plasma supernatant resting on top of the centrifuged cells, is typically placed on the analyzer and the tests performed, usually after automatic query of the test(s) required through interrogation of the LIS, and then test results are outputted from the analyzer to await verification. This process may be manual, or automated through a series of rules. In general, normal test results can usually be verified automatically, but the abnormal test results require a higher level of scrutiny. For any individual patient, the abnormal test result may actually be expected (e.g., on anticoagulant therapy), or else unexpected. In the latter case, the question arises whether the test result is truly reflective of the patient status, or is the result an artefact of a pre-analytical issue?
Modern instruments now come with several checks for pre-analytical problems, including the so-called HIL (hemolysis, icterus, lipemia) triad. In such cases, the instrument can usually overcome the spectral effects, and get an accurate test result reflective of the test sample; however, the instrument cannot account for any biological effects of HIL, and a recollection may still be recommended. In the case of potential sample clotting, causing a spurious coagulation test result, the usual check requires closer visual inspection of the sample, potentially using wooden sticks to physically check the sample for visual clots. This is a time-consuming process, and even if well performed may miss small clots, which could still compromise sample quality.
ML is considered as an extension of traditional statistics; usually, in the supervised approach, a computer program is trained from already classified (training) data to make predictions or decisions on unclassified (test) data. To assess its performance, a subset of data is used as the training set, and the remaining part (whose classification is hidden to the algorithm) is used to perform a simulation. Many models can be exploited to accomplish this task; each of them has theoretically defined advantages, but a practical comparison between algorithms is necessary in many cases. Fang et al. in their publication decided to use backpropagation neural networks (BPNNs), which is a widely used approach in neural networks classifiers. Fang et al. retrospectively retrieved from their LIS the results of coagulation testing, with 192 clotted and 2889 no-clot-detected (NCD) samples to form datasets for training and testing. Standard and momentum BPNNs were trained and validated using the training dataset with five-fold cross-validation, and then the predictive performances of the models were assessed on a testing dataset. The standard and momentum BPNNs could identify the sample status (clotted and NCD) with areas under the receiver operating characteristic (ROC) curves (AUCs) of 0.966 (95% CI, 0.958–0.974) and 0.971 (95% CI, 0.9641–0.9784), respectively. These represent extraordinarily high AUC values and indicate both high sensitivity and specificity for identification of clotted versus unclotted samples.
So, what does this mean, a method for identification or exclusion of clotted samples, and no longer need to visually inspect samples? Well, not just yet. As a caveat to the application of this process, the dataset comprised samples with five tests performed, being PT, APTT, thrombin time (TT), fibrinogen, and D-dimer. In other words, the model requires results from all five tests to enable the prediction to occur. In most laboratories, PT and APTT are the most-often performed tests, and TT, fibrinogen, and D-dimer are performed far less frequently, and generally in <10 percent of patients who have coagulation studies performed. Nonetheless, TT, fibrinogen, and D-dimer are often those tests that expert rules will reflex to in the case of unexplained prolongation of PT and/or APTT, and also with reference to patient information suggesting their potential utility. Thus, the work of Fang et al. should be seen as an important first step in the path toward an automated identification (or exclusion) of clots as the cause of unexpected abnormal coagulation test times. Further work can be looked forward to in this area over coming years.
POCT. In recent years, blood coagulation monitoring has become crucial to diagnosing causes of hemorrhages, developing anticoagulant drugs, assessing bleeding risk in extensive surgery procedures and dialysis, and investigating the efficacy of hemostatic therapies. In this regard, advanced technologies, such as microfluidics, fluorescent microscopy, electrochemical sensing, photoacoustic detection, and micro/nano-electromechanical systems (MEMS/NEMS) have been employed to develop highly accurate, robust, and cost-effective POC devices. These devices measure electrochemical, optical, and mechanical parameters of clotting blood.
The biggest appeal of POC devices is short measurement durations (25–45 min) compared to conventional coagulation tests (40–60 min). POC devices can also be used in pre-hospital emergency services to provide fast and reliable results for patients suspected of coagulation factor deficiencies.
Some of the recent technologies used to perform coagulation tests include electrical impedance spectroscopy, micro/nano-electromechanical (MEMS/NEMS) resonator-based rheometers, optical and photoacoustic measurements, and microfluidic viscometers.
Optical blood coagulation measurement techniques include laser speckle rheology (LSR), optical coherence elastography (OCE), fluorescent imaging, and surface plasmon resonance (SPR). While viscoelastic properties and shear modulus are correlated to the blood coagulation parameters in LSR and OCE, the fluorescent intensity level and refractive index are measured for studying clotting parameters in fluorescent imaging and SPR, respectively.
Advancements in coagulation testing
 Inderjeet Singh Bhatia
Inderjeet Singh Bhatia
Product Manager, Coagulation,
Transasia Bio-Medicals Ltd.
Coagulation tests help evaluate the blood-clotting mechanism in an individual. In recent times, the increasing prevalence of blood disorders, CVDs, and geriatric population are expected to contribute to the coagulation-testing market. Further, the wide range utilization of the D Dimer test during the COVID-19 pandemic is having a positive impact on the growth. By 2023, the Indian coagulation market is expected to reach ₹270 crore.
Developments in testing solutions. Coagulation tests are mainly performed by hospitals or attached labs, as compared to standalone labs. Routine parameters, such as PT and APTT contribute 80 percent of the tests. The diagnostics devices utilized to perform these tests have come a long way from quantifying optical density of clots in a cuvette to detection of clotting factors. The analyzers have gradually evolved by adding different technologies to the same instrument. These include optical detection of clot formation, chromogenic substrates, latex agglutination immunoassays and turbidimetry.
Further, manufacturers are focused on providing systems with high throughput, reliability, easy-to-use advanced software with LJ graphs and calibration curves for minimum errors. Advances in technology have resulted in greater capability, productivity, sensitivity, specificity, and ultimately improvement in the clinical care of patients. Transasia Bio-Medicals Ltd. offers semi- and fully automated coagulation analyzers and reagents that are innovative and technologically advanced. The product portfolio covers hemophilia profile, thrombophilia profile, D-dimer, calibrators, and controls.
Future perspectives. With increase in screening for coagulation and therapeutic monitoring, the market is witnessing a shift toward nex-gen POCT. The high cost of fully automated analyzers is bringing about this market trend. While large hospitals and lab chains with high workload continue to prefer fully automated systems, compact, cost-effective POCT devices are being preferred by labs with less workload. Also, AI- and machine-learning-based algorithms may very likely find place in the future novel POC blood coagulation measurement technologies.
The other upcoming technologies include T2 magnetic resonance, acoustic waves, laser speckle rheology, and infrared spectroscopy. With these technologies, the analyzers will offer advanced testing for Von Willebrand disease, platelet aggregation, heparin-induced thrombocytopenia, and chromogenic factor assays, D-dimer, and antiphospholipid antibodies.
The second category of the blood clotting measurement devices involves electromechanical technologies covering all types of MEMS/NEMS resonators, microfluidic viscometers, and ultrasound detection systems.
The third category is photoacoustic detection based on applying a laser beam and detecting the emitted acoustic signal (thermoelastic wave). In this technology, a photoacoustic signal enables monitoring of the adherent thrombi and emboli in deep tissue. As optoacoustic signals provide high-accuracy measurements of physiologic variables at depths greater than the optical diffusion limit, it is a powerful tool for recording the physical changes of tissue by monitoring the effective absorption coefficient, effective attenuation coefficient, and the thermoacoustic efficiency parameters.
The fourth and the most widely used technology for measuring clotting time in POC tests is electrochemical impedance spectroscopy technique since it is low cost and simple to implement. This technology employs integrated circuits, such as electronic amplifiers and digital synthesizers to measure impedance change of clotting blood with high accuracy. Second category of this technology is electrochemical aptamer biosensors for thrombin measurement.
Coagulation market trends
 Sanjay Patwardhan
Sanjay Patwardhan
General Manager – Sales & Marketing,
(A Beacon Group Company)
Coagulation has traditionally been measured by functional assays. Here anticoagulated plasma samples are mixed with a procoagulant and observed manually for clot formation. This technique provides accurate results by skilled operators. However, the increased demand for testing has led to the development of electrochemical, turbidimetric, and immunologic methods for use on an instrument.
From the first technique, developed by Dr Armand Quick in 1935, today coagulation testing has become one of the major IVD tests to provide answers in many clinical conditions, such as thrombophilia, hemophilia, liver disorders, and cardiovascular disorders. These are the major drivers for the increased need for coagulation testing. With PT and aPTT test being the most abundantly performed, today coagulation testing comprises of a bunch of tests like fibrinogen, thrombin time, D-dimer, factor assays, protein C and S, etc. With increased number of parameters, the need for instrumentation has also grown significantly.
The global coagulation market is projected to reach USD 5.0 billion by 2025 from USD 3.8 billion in 2020, with a CAGR of 5.7 percent between the period of 2020 and 2025. Clinical laboratories, hospitals, and POCT users were amongst the main target group for this fast-developing market.
The technologies used in coagulation testing by automation include use of fibrinometer that uses a pair of electrodes with a timing device. Measurement of turbidity, developed as a result of conversion of fibrinogen to fibrin fragments by using optical and mechanical measurement methods, is also widely employed in coagulation testing. Moreover, immunological and chemical methods are also in practice for the same purpose.
Technologies, such as microfluidics, electrochemical sensing, photoacoustic detection, and micro/nano electrochemical systems have also been used in developing coagulation testing by POCT arrangements. These POC testing setups are especially useful in detecting blood disorders, and for monitoring of anticoagulation therapies. The future trends would be employing multiplexed sensing using electrochemical biosensors embedded inside microfluidic chips that will lower the cost of testing multiple parameters in a compact-size analyzer.
Currently, working with single-channel coagulometers, Beacon group is all set to offer advanced systems in the most promising coagulation market for future needs.
Continued growth in POCT is certain as technologies improve and the benefits of POCT are realized in value-based healthcare. As more experience and understanding is gained by using POC devices, the benefits of quick results and ease of testing are likely to come to be seen as significant and desirable as well as routine.
Artificial Intelligence in diagnosis and monitoring. With the advancements in artificial intelligence (AI) and machine learning, algorithms that can track multiple parameters simultaneously throughout a treatment/diagnosis and find out patient specific patterns that can aid in pinpointing proper treatment or underlying causes will become one of the biggest developments in coagulation-measurement technologies in the upcoming years. Especially with the challenges set by the novel anticoagulant technologies for the current measurement techniques and the validity and applicability of diagnostic parameters, such as INR, simultaneous observation of multiple parameters, or complicated patterns within them, may be necessary for proper observation of patients with special needs. For these special cases, AI and machine learning-based algorithms may very likely find place within the future novel POC blood coagulation-measurement technologies in the upcoming years.
Although recent developments in the development of coagulation analyzers are promising, still there is a high potential for developing novel monitoring and therapeutic technology in the future, based on photoacoustic detection and nanotechnology.












