Urinalysis Instruments and Reagents
Unlocking the potential of new technologies
The focus is shifting back to where laboratory testing began so long ago, while simultaneously bringing urinalysis into the twenty-first century, thanks to new instrumentation technologies, new biomarkers, and new understanding of the value of urinalysis in contemporary medicine.
The microscopic analysis of urine has been one of the most routine inspection methods in modern hospitals at all levels, and it has developed to advanced microscopic technology and digital stage, but for the macroscopic characteristics of urine color, turbidity, odor, sediment, and foam, there are still not many ways to analyze it.
At present, the clinically common urine detection techniques include microscopy, urine analyzer detection and urine dry chemical detection. The advantage of microscopy is that the detection accuracy is relatively high, but the detection time is long and the work efficiency is lower, and the requirements for the inspection personnel’s operating technology and inspection skills are also relatively high. The advantages of urine dry chemical analysis and detection are simple and fast operation, and only a small amount of urine is needed to obtain a number of experimental parameters.
However, it has strong randomness, many interference factors, and more frequent shortcomings of false positives and false negatives. The principle of analysis and detection of urine sediment analyzer is basically similar to the principle of manual microscopy. Both are intuitive observations of the constituents of urine. However, the urine sediment analyzer undergoes strict timing, fixed speed, and quantification to quantify the test results, but the disadvantage is that the automatic urine sediment analyzer is susceptible to the influence of bacteria, myoglobin, and heat-prone enzyme factors, which reduces the specificity.
With the development of science and technology and the advancement of human understanding, the technology of urine testing has made great breakthroughs, but there is still a large distance from the ideal test target. The urine collection process is also affected by many factors, such as the patient’s collection time and improper operation during the collection process, resulting in test errors.
The complexity and diversity of urine components and the susceptibility of urine to various factors make the research of urine still a great challenge at present. The more factors that affect urine, the more samples are needed for research and analysis, and larger is the amount of sample needed. At present, people are more enthusiastic about blood research than urine. From the perspective of the number of papers published, urine biomarkers only account for 7 percent of blood, which also reflects the impact on urine. The research investment on urine is far less than the research investment on blood. These are the problems currently facing the further development of urine testing technology. Therefore, opportunities and challenges coexist.
Today’s laboratories face many challenges, causing a disruption in workflow and ultimately a delay in the delivery of patient care – this is especially true considering the challenges posed by the pandemic to laboratory workflow. A surge in coronavirus testing during the pandemic meant routine tests were put on hold. When it comes to urinalysis, the challenges are augmented as a routine urinalysis test is one of the tests most commonly ordered by physicians, representing up to 30 percent of all samples received.
Along with the operational challenge of adequate staffing, another hurdle that urinalysis labs face is the frequent need to manually review sediment. In urinalysis, there are generally two types of manual inspection procedures – microscopic review, which is performed under a microscope, and onboard instrument review, which is done within the analyzer.
In manual microscopy – the traditional method – urine is spun, and the sediment is observed manually through a microscope. Manual microscopic sediment examination is a labor-intensive, time-consuming process that lacks standardization in laboratories and can take up to six times as long per sample when compared to automated systems. Given that many of the results of such tests are negative, alternatives are sought to avoid the interruption of workflow, the resources required, and high overall system cost.
On-board instrument review can be conducted within the instrument and performed with the following particles – red blood cells, white blood cells, white blood cell clumps, bacteria, crystals, sperm, mucus, yeast, squamous epithelial cells, non-squamous epithelial cells, hyaline cast, and unclassified cast.
Conducting manual reviews creates bottlenecks of work, causes worker fatigue and impacts lab team morale. The key to successfully processing a large daily volume of samples is high throughput analyzers with reproducible capabilities, allowing for uninterrupted delivery of vital patient services in the event of instrument maintenance or unexpected downtime. Utilizing analyzers with automation, walkway, and remote IT technology should be a must in today’s labs so as to allow staff to focus their expertise across the entire laboratory, therefore, creating a seamless workflow experience.
The Indian market for urinalysis analyzers and reagents in 2021 is estimated at ₹214 crore. The growth took a major hit in 2020 since urinalysis has minimal role to play in Covid-19 testing or treatment. In 2021, the market saw a 19.94-percent increase over 2019.
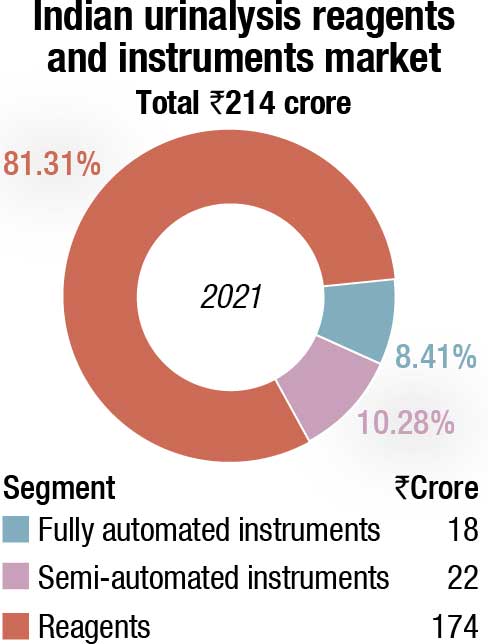
Reagents continue to dominate with an 80-percent share, valued at ₹174 crore.
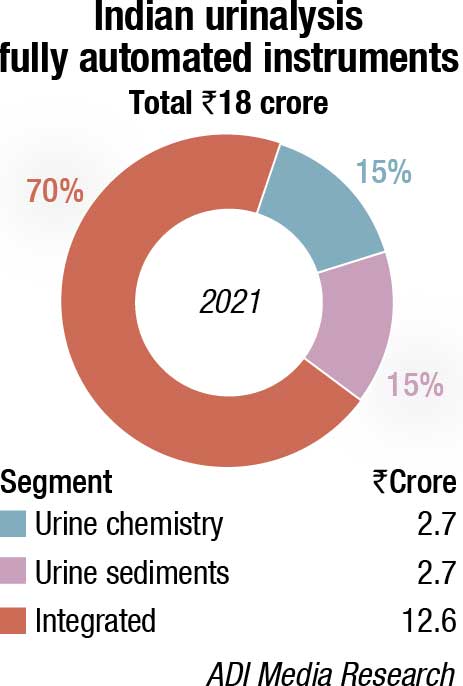
The fully automated analyzers segment is estimated at ₹18 crore in 2021. This is a 13.2-percent increase over 2020 and 7.4-percent increase over 2019. It is the integrated instruments that dominate with a 70-percent share by value.
The semi-automated instruments category, amounting to ₹22 crore, saw a 40-percent increase over 2020. The entry-level instruments constitute 70 percent of the market by value. The urine chemistry and urine sediments instruments contribute the balance share equally.
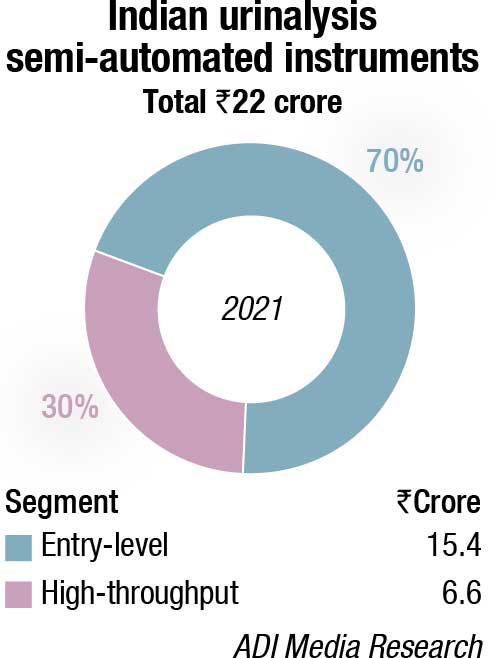
There is a distinct shift toward automation as the customer awareness has increased hugely in the last two years of Covid, and the documentation support required by NABL is possible only with fully automated instruments.
|
Major players Indian urinalysis market – 2021 Reagents |
||||
| FA reagents | Sysmex, Iris, Beckman Coulter, 77 Electronika (Suyog), Siemens, Dirui, YD Diagnostics, and Agappe | |||
| SA reagents | Transasia, Siemens, Dirui, Mission, SD, Agappe, Iris, and Rapha |
|||
| Fully automated instruments | ||||
| Segment | Brand | |||
| Urine chemistry | Dirui, Beckman Coulter, Rapid, Siemens, Roche, and Agappe | |||
| Urine sediments | 77 Electronika (Suyog), Sysmex, Dirui, Roche, URIT, and Beckman Coulter | |||
| Integrated | Sysmex, Dirui, Transasia, 77 Electronika (Suyog), Siemens, and Roche |
|||
| Semi-automated instruments (including strips) | ||||
| Tier I | Tier II | Tier III | ||
| Transasia | Dirui and Mission | Agappe, Iris, Rapha, and AVE China (marketed by Matrix Chennai) | ||
| *Vendors are placed in different tiers on the basis of their sales contribution to the overall revenues of the Indian urinalysis instruments and reagents market. | ||||
| ADI Media Research | ||||
The market is poised for a 20-percent annual increase over the next few years. The lab chains have included urine testing in the routine testing protocol, and are preferring to take the high-throughput option. Also, the reagents market is seeing a shift from visual strips to analyzer-based strips. After the acquisition of Thyrocare by PharamEasy, the sector is looking ahead to investment and acquisition by the Adani group.
The global urinalysis instruments and reagents market was valued at USD 3400 million in 2021 and is anticipated to progress at a 5.6 percent CAGR through 2028 to reach USD 4776.9 million by 2028. Market growth is driven by the increasing burden of urinary tract infections (UTIs), diabetes, and kidney and liver diseases and rising geriatric population and the subsequent increase in age-associated diseases and increasing adoption of POC diagnostic tests. The growing geriatric population in developed and developing countries is anticipated to boost the market demand. The increasing prevalence of diabetes, liver, and renal diseases with aging will significantly contribute to the demand for urine tests in diagnosis. Incidence of such chronic conditions among elderly population leads to higher frequency of prescriptions for tests like creatinine, albumin, glucose, ketones, and bilirubin, among others. According to the World Health Organization, the number of people aged 60 years and over is estimated to increase from 1 billion in 2020 to 1.4 billion by 2030. With increasing base of this age group, the industry is estimated to foresee tremendous growth. Additionally, higher risk of chronic kidney conditions with structural and functional changes in organs with aging will favor the testing volume.
The availability of refurbished instruments acts as a challenge in the growth of this market as these instruments offer the same functionalities as new equipment at a lower cost. Several end users, mainly small and medium-sized laboratories, opt for cost-effective and refurbished systems. In particular, the markets in developing countries, which are price-sensitive, prefer instruments that are cheap and offer similar functionalities. In the absence of well-grounded regulatory frameworks, there may be an influx of low-quality and refurbished products into the economy. Considering these factors, the demand for refurbished analyzers can be expected to increase in the coming years, as these systems offer the same functionalities at a lower cost. This is expected to hamper the revenue of companies offering branded analyzers, and thus restraining the overall growth of the market.
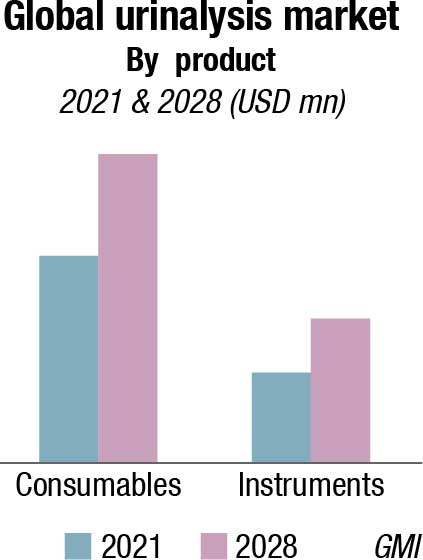
The urinalysis instruments market was valued at USD 1000 million in 2021, and is expected to reach USD 1528.4 million by 2028, with the increasing preference by healthcare professionals for automated urine analyzers. Product developments have improved the automation process in urinalysis, allowing accurate test results in case of complicated and serious illness. Additionally, infrastructure development in facilities leading to increasing adoption of such advanced instruments will strengthen the segment growth potential in the coming years.
The consumables segment is expected to have the major share in the global market and register a revenue of USD 3252.4 million by 2028. The frequent purchase of products including dipsticks, reagents, and disposables owing to their recurrent usage bolsters the market growth of this sub-segment. Furthermore, key factors including surging developments in multiple urine test kits and test strips, along with the constant demand for lower-cost and user-friendly consumables by end-users, are also fueling the market growth.
By application, the UTIs segment is anticipated to progress at a 5.2-percent CAGR through 2028 with the increasing incidence rate of urinary tract infections in developed and developing nations. For instance, according to the National Kidney Foundation, the UTIs lead to approximately 10 million physician visits each year in the US and are highly prevalent among women. Further, the prevalence of risk factors that possess high risk of UTI will contribute to the existing disease burden. Thus, growing availability and accessibility of diagnostic products across developed regions for early disease diagnosis will positively influence the segment demand.
The clinical laboratories segment held more than 31-percent revenue share in 2021, and is anticipated to witness substantial CAGR, based on end-use. As clinical laboratories offer various advantages that help in determining a course of action or treatment for a patient, the facilities are anticipated to foresee increased volume of urinalysis that will contribute to market expansion. Additionally, collaboration of clinical laboratories and hospital settings for various diagnostic tests to reduce cost, time, and human resources is expected to impel the urinalysis market growth.
Moreover, availability of skilled administrators and specialists in settings will drive the customer preference toward clinical laboratories.
The global market consists of numerous players. Few of the prominent market players operating in urinalysis industry are Sysmex Corporation, HORIBA, F. Hoffmann-La Roche Ltd., ACON Laboratories, Inc., Cardinal Health, Abbott, Bio-Rad Laboratories, Inc., ARKRAY, Inc., Erba Mannheim, Siemens Healthineers, Teco Diagnostics, and 77 Elektronika Kft, among others. These market players are actively involved in product development and strategic collaborations to expand their business reach.
In October 2021, Olive Diagnostics, a home-based urinalysis technology developer, began a funding round supported by the Maccabi Foundation for the world’s first hands-free toilet optical sensor targeting early disease detection in the elderly and pregnant women.
Automation leads the way to standardization in urinalysis
 Grieston Fonseca
Grieston Fonseca
Dy. Manager – Urinalysis,
Transasia Bio-Medicals Ltd.
In clinical laboratories, urine analysis is a major screening and a common diagnostic test in addition to blood and sputum analysis. It provides important information to help diagnose and monitor renal and UTIs. Physical, chemistry, and sediment analysis provides information on difference parameters, such as pH, uric acid, protein, glucose, bilirubin and presence of RBC/WBC, casts, cells, or particulate matter, among others. However, various factors can influence result interpretation by both visual and microscopic examination. It is thus important for laboratories to bring into practice standardized guidelines to eliminate the chances of errors.
Need to standardize the pre- and post-analytical phase of urinalysis. It is important to focus on the pre-analytical phase in order to improve the reliability of test results and provide efficient patient care. Though modernization of sampling techniques has contributed to a reduction in errors, even today, the lion’s share of errors in urinalysis lies outside the analytical phase, both pre- and post-analytical continue to remain vulnerable. Patient preparation, sample collection, transportation, and preservation can be important potential sources of error. Individual perception, lighting, and proper reporting of observations are all steps that need to be standardized.
Automation for standardization of analytical processes. The sediment or microscopic analysis is also prone to various subjective differences arising from the volume of sample taken, centrifugation time and speed, re-suspension of sediment in supernatant, and the number of view fields observed. In addition, the reporting formats differ between laboratories and that may result in variations during result interpretations.
Automation of urine chemistry and sediment analysis greatly standardizes objective evaluations, thereby reducing the number of errors and improving result reproducibility. Automated aspiration, processing, and evaluation not only minimize manual effort but also reduce the time taken. Photometric evaluation of the test strip in the semi-automated urine chemistry strip readers avoid subjective challenges and reduce errors.
Recent advancements in automation. Recent advancements in fully automated urine chemistry and sediment analyzers allows for complete walk-away systems, resulting in improved efficiency. Automated urine analyzers are especially beneficial for high-workload laboratories since they can simultaneously perform microscopic analysis for a large number of samples, thereby improving TAT.
Further, in the case of digital microscopy-based sediment analyzers, the availability of high-resolution sediment images for review, as well as the option to save and store them, has remarkably advanced the scope of testing.
In September 2021, at the AACC Annual Scientific Meeting & Clinical Lab Expo, Beckman Coulter unveiled DxU Iris Workcell, a revolutionary urinalysis solution that reduces the sample processing time.
Urinalysis – Automation is leading the way
 Shobhit Jain
Shobhit Jain
Product Manager – Biochemistry, Urinalysis, Marketing Events, and Branding,
Sysmex India Pvt. Ltd.
Urine analysis comprises of physical, routine, and microscopic examination. It is an integral part of clinical laboratories, and one of the most-performed diagnostic tests. This simple, noninvasive test is a potent screening tool for clinicians, which can provide as much as possible information on the status of renal, urological infection, and metabolic function in the body. Apart from routine screening, it may also be helpful in uncovering the status of the disease that may not be showing significant signs or symptoms.
A lot of vital information can be obtained through urine examination; still, its clinical utility is affected to a great extent by limitation of its conventional manual methods including bio-chemical and microscopy. Biochemical analysis has improved with reagent strips, but challenge remained in the microscopic part. Other challenges like non-standardization of process, person-to-person variation, centrifugation, and slide preparation needed a whole automated system in place with minimum human involvement and maximum automation.
In the last decade, there was a paradigm shift in laboratory operations, and now automation is leading the way for urinalysis testing. Technological advancement in biochemical analysis and microscopic examination in urinalysis has helped to set and standardize the process. Automation has changed the way of testing, reporting, and documentation of laboratories with improved quality and lowered TAT. Some of the key features of automation in urinalysis are standardization, quality control, added clinical values, data management, accuracy, and reproducibility.
Automation in urinalysis can be further categorized in bio-chemical analysis and particle analysis (microscopy). In bio-chemical analysis, CMOS sensor has played a great role in identifying the strip pad position, optimizing detection area, and correcting abnormal coloration. This cutting-edge technology has eliminated all possible errors due to manual process. Similarly, for particle analysis, fluorescence flow cytometry technology has great advantage as it can recognize specific and unique properties of different particles by size, labeling DNA, complexity, and specific features, and so determining the particle type. Also, high accuracy of bacteria counts and accurate differentiation in RBC morphology (isomorphic and dysmorphic) is helping in faster diagnosis and better patient care. These are the features available in Sysmex UN series, which is integration of sediment, chemistry, and also scalable based on customer need. Looking at the facts, it can be concluded that the future of urinalysis lies in automation.
In February 2021, 77 Elektronika Kft. announced the launch of LabStrip U mALB/CREA urine test strips. The product is intended for the rapid and semi-quantitative recognition of creatinine and albumin compounds in native urine. Such developments are expected to strengthen the company’s product offerings in the emerging markets.
In January 2020, Sysmex Corporation announced the official agreement to invest in Astrego Diagnostics AB, Sweden-based rapidly growing in-vitro diagnostic company.
New technological advances have paved the way for significant progress in automated urinalysis. Time and accuracy are the two key factors for diagnosis. In UTIs, urine dipstick is very fast and easy to use, but it lacks the accuracy, whereas on the other hand, urine culture for antimicrobial susceptibility testing shows clinically reliable and accurate results, but it takes up to 3 days to give results. Many novel and improved diagnostic technologies and tools are introduced in the market, and some of them are already approved for clinical use and helped significantly in increasing the accuracy and decreasing the time of the test; a good example would be nucleic acid tests and mass spectrometry. Some other technologies show promising future, such as the utilization of smartphone for urinalysis.
Recently, a new generation of analyzers that integrates and automates the two primary functions – chemistry and microscopic particle analysis – have been introduced. Automated microscopic and strip analyzers have been combined into fully automated workstations. These automated solutions are gaining popularity, which offer the phase-contrast images to classify the cells that are difficult to recognize. Microscopic images can be viewed in three different optical modes – bright field, phase contrast, and composite, a synthetic one. With the help of the stored images, the lab technician can use the system for new personnel training. With the phase-contrast option, particles like isomorphic, dysmorphic, and acanthocytes can be easily identified.
Urinary flow cytometry and UTIs. Urine culture is considered the gold standard for UTI diagnosis. It can determine the level of bacteriuria and antimicrobial susceptibility. However, there is no standardized bacterial count indicating significant bacteriuria, applicable for all types of UTIs. Scientific evidence supporting current urine culture guidelines is often incomplete, and in some cases, the guidelines do not indicate a clear choice. Because of the high percentage of negative results, there is a need for an efficient screening method, reducing the number of unnecessary culture tests. Several methods for screening-out culture-negative samples have been developed, including dipstick chemical tests (nitrite, leukocyte esterase, urinary protein, and urinary hemoglobin) and manual or automatic microscopic examination of urine sediment (detection of particles, WBCs, and microorganisms). Although these screening methods are primarily used in general practice and microbiology laboratories, they are subjective and time-consuming and demonstrate poor sensitivity and negative predictive value.
Many authors have reported using flow cytometry to detect bacteria and WBCs in urinary samples. Flow cytometry can reduce the number of samples cultured, with a substantial decrease in workload, time, and costs, especially in clinical laboratories. Using flow cytometry, negative results could be reported earlier, substantially reducing unnecessary empirical antibiotic prescriptions. The use of flow cytometry can reduce the number of urinary samples processed in the clinical laboratory by 28–60 percent. However, there is a wide variation in the applied cut-offs, as well as in the sensitivity and specificity of the obtained results in the literature. These variations are mainly owing to the spectrum of clinical conditions of the patient populations enrolled in these different studies. These differences can be attributed to the different definitions used to classify UTIs, which depend on the guidelines applied in a specific setting. Therefore, the applicability of flow cytometry to screen for negative urine samples strongly depends on population characteristics and the definition of a negative urine culture. In addition, a limitation of automated urine analyzers compared with culture is that they count both live and dead bacterial particles, yielding higher particle counts.
Although dry chemistry technology for urinary test strips has made limited progress, advances in electronic detection have considerably improved the analytical sensitivity of test strip readers over the years. Major improvement in the test strip technology has been made in recent years. Not only highly sensitive test strips are being introduced, but also now, one can find strips, which give quantitative results for urinary proteins. The financial aspect is also of great importance, especially in the Third World and developing countries; inexpensive test strips for various diagnostic reasons, such as the diagnosis of diabetes from urine sample, are available. Test strip method also shows promising result in antibiotic susceptibility tests; if optimum diagnostic requirement is reached, it can reduce the test time significantly from 2 to 3 days to a few hours.
Using CMOS technology, very-sensitive readings can also be obtained for leukocyte esterase and peroxidase activity. Similar to albuminuria, the reflectance data can be used for quantitative analysis. In parallel, the use of sensitive dyes has improved the sensitivity of albuminuria test strips. An interesting recent evolution is the use of smart phones for reading and interpreting urine test strip results. Mobile healthcare platforms have been proposed, combining a pocket-sized colorimetric reader and commercially available 10-parameter urinalysis paper strips, capable of sending data via a smartphone.
Optical microscopy allows for direct visualization of urinary sediments for morphological characteristics but is time-consuming and prone to errors; the true alternative to microscopy is fully automated urine analyzers.
Principle. Analyzers read urine sedimate by flowcytometry or digital imaging with auto particle recognition or automated microscopy with digital imaging by flowcytometry. Uncentrifuged urine is categorized according to its fluorescence, size, impedance, and forward-scattered light. Microscopy with digital imaging produces a monolayer of urine sediment by centrifugation in a special curvette and is analyzed by bright field and phase-contrast microscope. Usually, 15 fields are analyzed. Auto detected are WBC clumps, hyaline casts, pathological casts, squamous epithelial cells, non-squamous epithelial cells, bacteria cocci, bacteria rods, yeast, mucus, sperm, crystals, calcium-oxalate monohydrate, calcium-oxalate dehydrate, uric acid, triple phosphate, RBC-g1, ghost RBC flags, and AMO deposit. A high number of samples are examined in a short time with small volume and acceptable accuracy, leaving time for manual examination of more complex samples. Future of sediment technology is urine analyzers. Dr Anuradha Sekaran, Director & Chief of Pathology, Asian Institute of Gastroenterology & AIG Hospitals, Hyderabad
In view of the great need for the development of portable and cost-effective readers, pocket-sized colorimetric readers can be combined with dipsticks in a device that is able to transmit digital information via a smart phone, offering an integrated solution for detecting disease in areas with limited access to trained experts. Advances in microfluidics have enabled the development of new chip-based assays, which will alter the field of automated urinalysis in the near future. Alongside conventional urinalysis applications, integrated microfluidic chips have been described as a promising tool for measuring the concentration of bladder cancer cells in urine samples. Similarly, microfluidic paper analytical devices have been designed and fabricated for evaluating bacteria known to cause UTIs (Escherichia coli) and sexually transmitted diseases (Neisseria gonorrhoeae) in human urine samples.
Automated urinalysis has come a long way in the last two decades in terms of technology. Microscopy and flow cytometry-based devices both produce therapeutically valuable results, and automated test strip reading adds to the usefulness. Integration of existing technologies may help to reduce turnaround times even more.
Urine biomarkers have the advantage of early diagnosis of diseases. Urine biomarkers have the advantage of early diagnosis of diseases, because in the case of no obvious pathological symptoms in the early stage of the disease, the homeostasis mechanism effectively maintains the stability of the body’s internal environment and removes harmful ones from the body through various methods. Keep the composition and characteristics of body fluids (especially blood) unchanged or within the normal range. Therefore, the early characteristics of many diseases are difficult to reflect in the blood. Compared with blood, urine has no homeostasis mechanism, and urine can adapt to subtle and comprehensive changes, especially in the early stages of the disease. Many early disease markers in urine appear earlier than in the blood, and even earlier than the patient’s symptoms, signs, and imaging pathology examinations. With the latest advances in the discovery of nano-scale extracellular vesicles called vesicles, urine vesicles have become a new source for screening and characterizing potential biomarkers. At present, a new on-disk laboratory platform has been developed. It is used to quickly and effectively isolate exosomes from urine, and potential biomarkers have been discovered, which can be used for early screening of bladder cancer. In the near future, further research on urine biomarkers will greatly promote the early diagnosis, prevention, treatment, and prognosis of various diseases. Compared with plasma biomarkers, urine biomarkers are used as disease biomarkers. The early stage showed many advantages and deserves more attention and further research.
Timely results help in good patient management
 Rajesh Maheshwari
Rajesh Maheshwari
President and CEO,
Suyog Diagnostics Pvt. Ltd.
On-time results play an important role for urinary tract infection (UTI) patient management (constitute 70% of tests in a microbiology lab) and in more important critical conditions, where infections of the central nervous system (CSF culture) plays an important role in quick diagnosis and treatment.
It is also important to rule out sepsis, multidrug resistance issues, which are real worrying factors and cause of concern for every hospital. They need to keep these infections from spreading. Quarantine, dedicated hospital staff, and a separate room for each patient is a huge expense.
A preliminary screening to test for indicators of UTI, like pus cells, bacteria, mucus, nitrite, epithelial cells and leucocytes, and dismorphic RBCs, which play an important role in kidney health, testing equipment like the UriSed 3 Pro with complete microscopic images by cuvette-based sedimentation technology having both bright field and phase contrast. And the LabUMat 2 gives the result accurately and quickly. It also helps to confirm negative or positive UTI results.
A complete chemistry and microscopy parameters cater to both the pathologist and the microbiologist. Large numbers of samples can be easily handled within hours.
The HB & L culture system with its fast turn around time (TAT) helps reduce the burden of negative samples; a complete solution for three classes of patients – pediatric below age of 2 years, patients on catheter, and pregnant women – who require quick results for UTI management can benefit from this technology.
For critical samples like CSF, other body fluids like ascitic, pleural, synovial, and peritoneal fluids are tested for severe infections.
It greatly reduces the stress of both the clinician and the patient on receiving a negative result in a few hours and positive by reducing time against conventional method.
Dedicated media also help detect MDROs in a few hours.
-
Light scattering technology;
-
Quantitative results expressed in CFU/mL; and
-
Real-time detection of bacteria-growing curves.
To top it, a quick accurate identification in a few minutes will be ideal for any patient management – reagent-free, no sample preparation, no pre-treatment, and no maintenance.
Prospects and foreground for the future development of urine testing. Urine is rich in biological information. In the era of rapid development of bioanalysis technology and big data processing information, the human health code, contained in the complex urine components, will also be continuously decrypted. Some researchers believe that the method of searching for cancer DNA in body fluids rather than blood may be more widely used. Now for colon cancer, prostate cancer, and other new-generation urine diagnostic technologies, and some signs of finding signs in urine the better than the markers in the blood has also been initially recognized and explored by people. The technology of detecting cancer information markers through urine has entered the development process or will soon enter the hospital. Some scholars also want to use urine to detect volatile organic compounds to achieve the purpose of distinguishing cancer types. Some experiments have proved that the health of athletes can be monitored through urine observation and analysis, so as to protect the health of athletes and improve their physical fitness, which also indicates whether ordinary healthy people can also perform health self-examination through urine. Accessibility makes it easier for people to monitor their physical health.
Today, with the continuous development of medical inspection technology, these are all possible ideas. If more researchers can participate in the mining of the potential gold mine of urine testing, it may greatly accelerate the development of medical laboratory science and change the face of medical research and medical practice in the next century.











