Microbiology Instruments and Reagents
Clinical utility of advanced microbiology technologies
There remains disconnect between clinicians and some payers and hospital administrators in terms of understanding the potential clinical utility of these novel technologies.
Advanced microbiology technologies are rapidly changing the ability to diagnose infections, improve patient care, and enhance clinical workflow. These tools are increasing the breadth, depth, and speed of diagnostic data generated per patient, and testing is being moved closer to the patient through rapid diagnostic technologies, including point-of-care (POC) technologies. While select stakeholders have an appreciation of the value/importance of improvements in the microbial diagnostic field, there remains disconnect between clinicians and some payers and hospital administrators in terms of understanding the potential clinical utility of these novel technologies. Therefore, a key challenge for the clinical microbiology community is to clearly articulate the value proposition of these technologies to encourage payers to cover and hospitals to adopt advanced microbiology tests. Specific guidance on how to define and demonstrate clinical utility would be valuable. Addressing this challenge will require alignment on this topic, not just by microbiologists but also by primary-care and emergency room (ER) physicians, infectious diseases specialists, pharmacists, hospital administrators, and government entities with an interest in public health.
Indian market dynamics
The Indian market for microbiology instruments and reagents in 2018 is estimated at Rs 425 crore. bioMérieux and BD India continue to dominate the market, with BD dominating the reagents market and bioMérieux the instruments segment. Hi-Media is aggressive in non-instruments-based reagents.
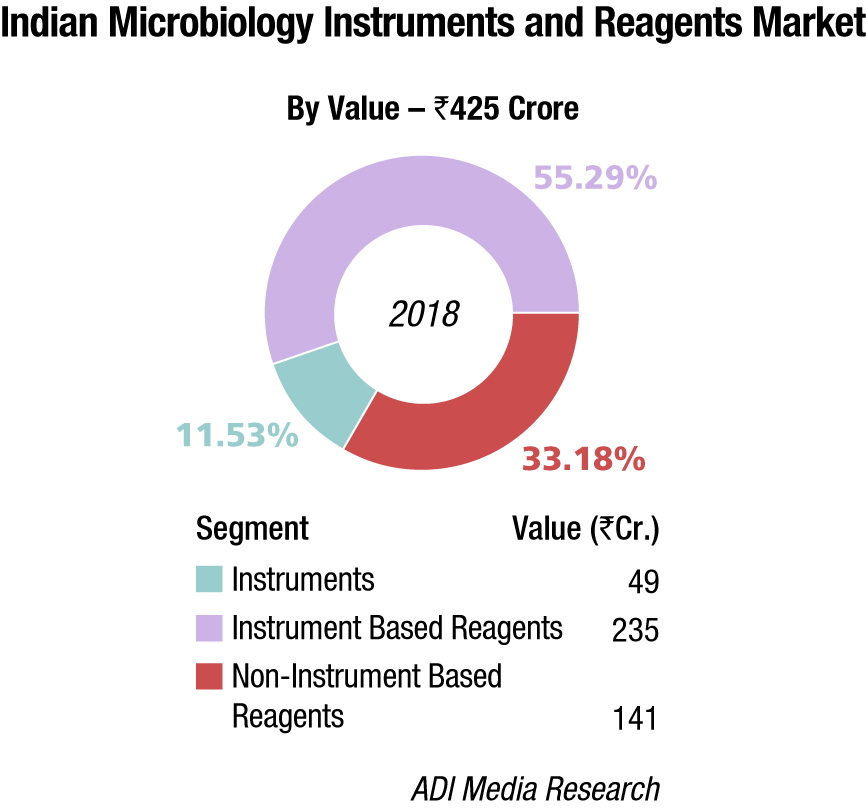
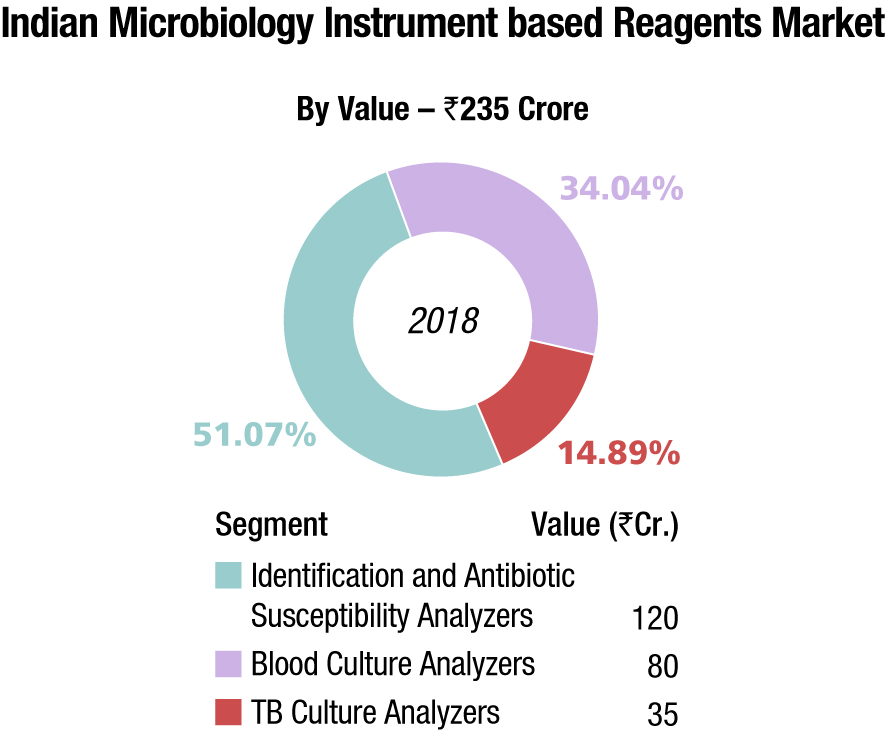
The instruments-based reagents were Rs 235 crore in 2018, a 55.29-percent share of the total market, non-instruments reagents at Rs 141 crore, at a 33.18-percent share, and the instruments at Rs 49 crore, at 11.53 percent.
The instruments are estimated at Rs 49.2 crore, with 610 instruments sold in 2018. The market was dominated by identification and antibiotic susceptibility analyzers at 57.37 percent share. With an increased penetration of molecular platforms, the TB culture microbiology testing is on a gradual decline. In 2018 too, the market for both TB culture analyzers and TB instrument-based reagents declined. IDST testing dominates with the instruments commanding a 57.37-percent share, by volume, and 30 percent market share, by value, and the instruments-based reagents at 51 percent.
In India, the level of automation in the microbiology laboratory has been lagging behind that of other major clinical laboratory segments, such as chemistry and hematology. The slow acceptance of the technology is in part due to the complexity of developing automation suitable for microbiology tests.
The introduction of automated microbiology instrumentation has been delayed by a number of intrinsic and technical problems. The diffusion of automated microbiology systems, once the technology was developed, has not matched that of other automated laboratory technologies. The acquisition of automation in microbiology has been slowed by forces less easily identifiable than the effects of various reimbursement plans. Automated microbiology is fast, but the costs per test, and initial capital investment are quite high. The research in microbiology should focus on fast, easy-to-use, rapid tests for bacterial, viral, and parasitic pathogens, such as multidrug-resistant organisms. Thus, a faster turnaround time (TAT) and immediate results are the major factors that will drive the microbiology market. Another important factor to be considered is to use biological reference materials that are authentic, traceable, and reliable to ensure the quality control of tests being performed. The need of the hour is to combine advanced IT solutions like artificial intelligence and nanotechnology with the existing diagnostic technologies to develop rapid, cost-effective tests, which can be easily interpreted and made available across the entire country.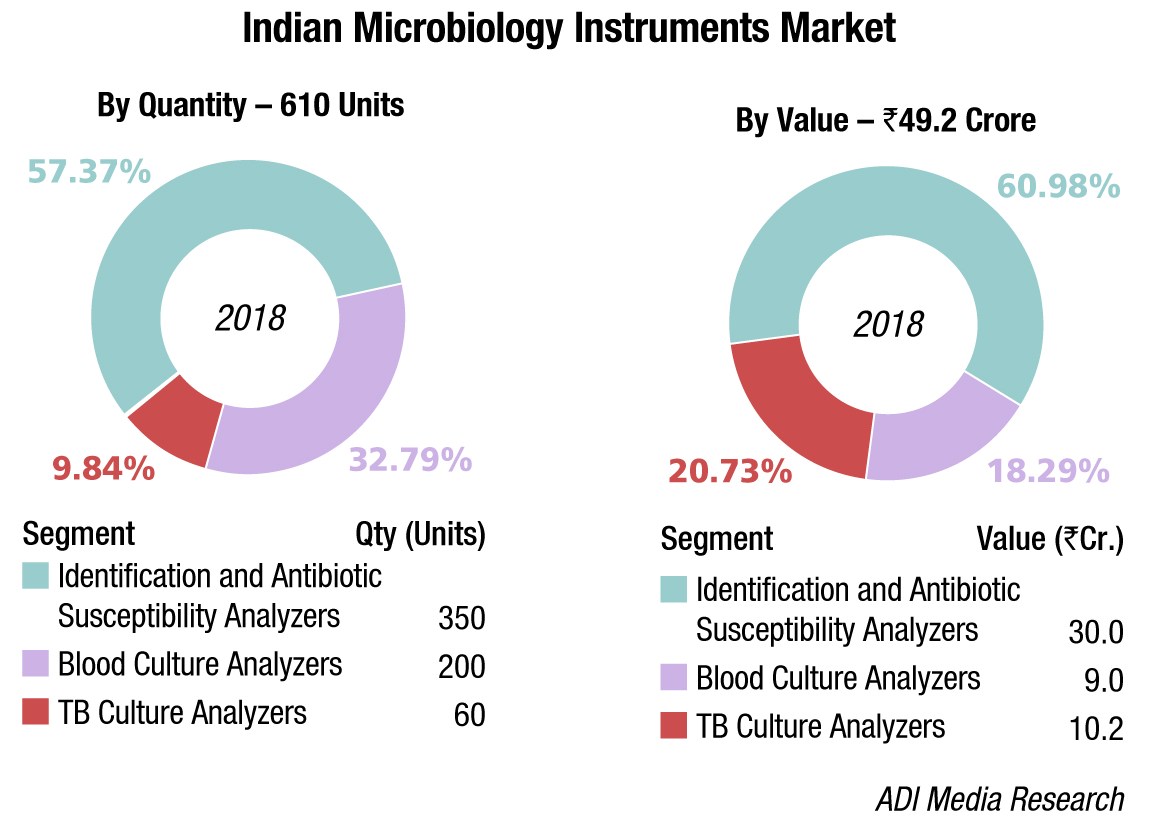 Some laboratorians still believe that current instrumentation is not the ultimate technology and expect better automation on the horizon.
Some laboratorians still believe that current instrumentation is not the ultimate technology and expect better automation on the horizon.
The driving force behind the need for rapid reporting of microbiological test results is the clinical relevance in a time of financial austerity, a time when cost and healthcare effectiveness to the patient and diagnostician looms ever larger, and where after-the-fact results at high expense are coming under severe scrutiny worldwide.
| Segment | Vendors |
|---|---|
| Indian Microbiology Automated Instruments Market | bioMérieux, BD India, Beckman Coulter (Siemens products), Thermo Fisher, and Trivitron |
| Indian Microbiology Instruments-based Reagents market | bioMérieux, BD India, Beckman Coulter (Siemens products), and Thermo Fisher |
| Indian Microbiology non Instruments-based Reagents market | Hi-Media, BD India, Thermo Fisher (oxoid), Bio-Rad, and Microxpress (Tulip Diagnostics), bioMérieux, Beckman Coulter (Siemens products), Merck Millipore, and Titan Biotech |
Global market dynamics
The global clinical microbiology market is valued at USD 2.6 billion in 2018 and is expected to reach USD 3.98 billion by 2025, with a CAGR of 6.26 percent over 2019–2025, predicts Fior Markets. Increasing need for screening for analyzing sickle cell disease, specifically in newborns, is anticipated to drive the growth of global clinical microbiology market.
The clinical microbiology market witnessed a huge demand in the past few years, mainly due to the rising cases of infectious diseases, increased demand for microbiology testing, and rapid adoption of automated testing methods in laboratories to identify the certain cause of disorders in patients. The global burden of infectious diseases including HIV/AIDS, malaria, and neglected tropical diseases is nearly 63.40 million and 66.82 million respectively. Additionally, both public and private sectors are increasingly making a huge investment to detect the health-related issues, which in turn fosters the adoption of clinical microbiology. For example, The National Institute of Health (NIH), a part of the US Department of Health and Human Services, is one of the medical research agencies that have made many innovations regarding microbiology testing that improve health and save lives. Furthermore, technological advancements, such as molecular diagnostics, digital microbiology, and mass spectrometry, changing the way of implementation of rapid diagnostics techniques is also supplementing the market of clinical microbiology. However, lack of trained manpower and government regulatory policies are turning out to be the restraining factor of this market.
Technological advances
The clinical microbiology laboratory has historically been considered low-tech, especially when compared to the clinical chemistry laboratory. However, systems are emerging for the clinical microbiology laboratory with the potential to automate almost all areas of testing, including inoculation of primary culture plates, detection of growth on culture media, identification of microorganisms, susceptibility testing, and extraction and detection of nucleic acids in clinical samples. As a result, the workflow in the microbiology laboratory is changing at a rapid pace and microbiologists have the challenge of selecting the most appropriate, clinically useful, and cost-effective automation for their laboratories.
Nucleic acid amplification, including PCR and TMA. Nucleic acid amplification, using thermostable polymerase (PCR), was initially reported in 1988, and this method remains largely unchanged as it forms the backbone of molecular diagnostics in clinical microbiology laboratories today. Properties, such as high sensitivity and specificity, an extremely low limit of detection (1 to 10 copies of the target), and rapid results, have led to proposed changes in the definition of the gold standard method for detection and identification of microorganisms in clinical specimens, especially for those that are difficult to culture, including fastidious bacterial or viral pathogens (7–10). While the basic principle of nucleic acid amplification tests (NAATs) has not changed, technologies surrounding this core, including amplification strategy, amplicon detection, multiplexing of reactions, and automation of the entire process into sample-to-result platforms, have provided a large menu of options for the molecular microbiology laboratory to choose from. One such modification is the departure from PCR-based amplification to transcription-mediated amplification (TMA) of a nucleic acid target. This method differs from PCR in that the target sequence is typically an RNA molecule (mRNA or rRNA), which may be present at a high copy number in the cell. Reverse transcriptase and engineered oligonucleotide primers are used to simultaneously generate a cDNA template and incorporate a promoter sequence recognized by a highly efficient, phage-encoded RNA polymerase enzyme.
LAMP and HDA technologies. To maximize the benefits of molecular testing, developers of diagnostics have begun to focus on technologies that employ both simplified technology and simplified specimen preparation in an attempt to bring molecular assays closer to the patient. These technologies have the potential to further reduce TAT, which may positively impact patient care and reduce the overall cost of healthcare. Isothermal amplification methods, including loop-mediated isothermal amplification (LAMP) and helicase-dependent amplification (HDA), effectively remove the need for expensive thermocyclers and technical optimization of cycling conditions. These methods can be coupled to alternative detection technologies (i.e., fluorescent probe-independent detection methods) that eliminate the need for sophisticated optics. This further reduces the cost of instrumentation and enables these tests to be used outside today’s molecular laboratory and closer to the point of care (POC).
Outlook
The need for improvement in microbial diagnostics and thereby in management of infectious diseases is clear and urgent. This need has the potential to be filled by a combination of new technologies that have entered the diagnostic space or will enter it shortly. However, there is a clear gap in the field that is preventing these technologies from being widely deployed to fill the current unmet clinical need for rapid and improved testing. While the necessity of deploying better microbial diagnostics is not lost on microbiologists and infectious diseases specialists, other key stakeholders have lower awareness. Therefore, a collective effort is needed from microbiologists and clinicians handling infectious diseases to communicate to other stakeholders the costs and downsides of the current standard of care (SOC). Demonstrating and communicating how the low cost of phenotypic methods is often offset by the high cost of preventable morbidity and mortality that comes from a slow diagnostic SOC, and how new tests can directly impact and improve clinical decision making, is needed. Clearly defining and describing these issues to commercial payers, hospital administrators, and government regulators will smoothen the deployment of these technologies and benefit individual and communal health.












