MedTech
For ventilators, it’s a complete shift!
As COVID-19 progressed, the dynamics changed completely. Ventilators were not the savior as perceived to be earlier.
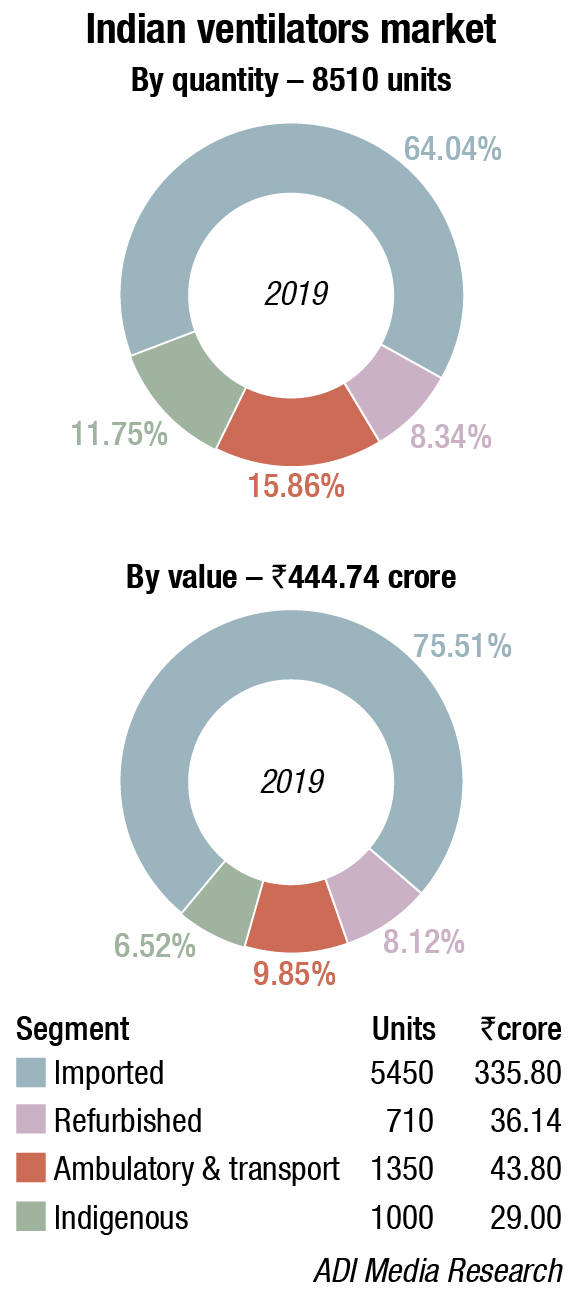
When the first wave of coronavirus patients flooded the hospitals earlier this year, clinicians were heavily focused on ventilators. At the apex of the pandemic, one in four people hospitalized for COVID-19 needed these machines to breathe, and the supplies were running short.
Six months later, the picture changed dramatically. Ventilators are still critical for some patients, 10 percent percent of those hospitalized earlier this week depended on artificial respiration, but clinicians now try to employ less invasive protocols first, like high-flow oxygen or repositioning patients to ease breathing, referred as proning.
The medical teams moved from ventilators to proning, through temporary detours like hydroxychloroquine, remdesivir, and dexamethasone. In recent months they are against the use of chloroquine or hydroxychloroquine and certain monoclonal antibodies (sarilumab, tocilizumab, and siltuximab), citing a lack of data on their efficacy and safety. In September, some also opposed the use of convalescent plasma for the same reason.
Down memory lane
In May 2020, the Technical Committee constituted by the Empowered Group-3 that had been created under convenorship of Dr P D Waghela, Secretary, Pharmaceuticals, MOHFW projected that India would need at least 75000 ventilators till June 30, against 19398 units available in the country then. The PM CARE Fund allotted `2000 crore.
HLL set out to procure 60884 ventilators, with unit prices ranging from `150000 to `400000, to be supplied by end-June. Orders were placed for 59884 ventilators on domestic manufacturers and 1000 units on overseas manufacturers.
The major domestic players included Skanray (in collaboration with Bharat Electronics Limited) for 30000 ventilators, AgVa (in collaboration with Maruti Suzuki Limited) for 10000 ventilators, AP Medtech Zone for 9650 ventilators and Allied Medical 350 machines.
The auto industry, PSUs, and defense laboratories were invited to contribute to ventilator supplies. In turn, the regular manufacturers enhanced their production capacities. Ventilator capacity went up from about 300 units to 30000 units per month and from eight manufacturers to 16 manufacturers. For instance, AgVa expanded its manufacturing capacity to 60000 units, Trivitron Healthcare to 60000 units, and Skanray to 120000 units per annum. International players too pitched in. Medtronic open-sourced the design specifications on its portable ventilator, Puritan BennettTM 560.
 “Ventilators play a significant role in reducing the fatality rate of COVID-19; it is wonder drug in the treatment of COVID-19 patients. Therefore in severe and critically severe patients with symptoms of respiratory difficulty or failure, ventilators need to be used to assist breathing and ensuring oxygen supply to brain and other vital organs. Mechanical ventilation is one of the major methods in the treatment.”
“Ventilators play a significant role in reducing the fatality rate of COVID-19; it is wonder drug in the treatment of COVID-19 patients. Therefore in severe and critically severe patients with symptoms of respiratory difficulty or failure, ventilators need to be used to assist breathing and ensuring oxygen supply to brain and other vital organs. Mechanical ventilation is one of the major methods in the treatment.”
Dr Kalpana Goel
Senior Consultant and Head of Department, Department of Anesthesiology, Venkateshwara Hospital
As COVID-19 progressed, the dynamics changed completely. Ventilators were not the savior as perceived to be earlier. Non-invasive oxygen delivery through nose prongs, using non-invasive ventilation (NIV) or BiPAP mode, was sufficient in most cases. On the contrary, ventilators were seen as harming the patients. Delhi’s COVID-19 committee reported an 85-percent mortality rate among COVID-19 patients who were put on ventilators in the Capital, as did Bangalore Medical College and Research Institute.
There was mayhem as the government went ahead and changed its specifications for ventilator manufacturers, like asking for BiPAP mode and a high-flow nasal cannula with a flow rate of 100 percent. In addition, exports were banned and states were not allowed to buy directly from the makers. Excess stocks piled up with the manufacturers and they inadvertently landed in a major funds crunch. And the ventilators, that had been procured were being grossly under-utilised.
An article authored by Vishwaprasad Alva, Managing Director, Skanray Technologies, Oct 11 2020, Deccan Herald says it all. Excerpts reproduced
“Come COVID-19, and the only choice before the government was to scout for domestic manufacturers, and Skanray and Max Ventilators of AB Industries were the only ICU ventilator manufacturers with long domain experience. The rest of the ventilator story is there in overdose and variety in public domain: Start-ups, joint ventures, cheap imports, second-hand machines, scams, accolades, theories and metaphysics, all emerged during this period, all claiming to be making or supplying ventilators. Our politicians congratulated each other for creating products for COVID-19 in a short time. The truth is, we always existed and were exporting our machines while the government slept over this critical healthcare sector and continued to import ventilators for reasons well known to all of us who deal with government.
On March 18 this year, we got calls from NITI Aayog, Health and Defence ministries requesting 100,000 ventilators to deal with the COVID-19 calamity. The underlying story here is that the Centre and the states could not import from China or elsewhere during COVID-19 since these countries had domestic shortages and compulsions of their own. China, which had dismantled COVID-19 hospitals in April, had lots of sparingly used machines ready to export, but it chose to sell them to European and American markets than India for higher profits. Still, not many people know, our MedTech imports from China have grown significantly since March while we have all been screaming Atmanirbhar Bharat and Make in India from our rooftops. Most Indian manufacturers suspect that whatever domestic buying is happening now will end as soon as the pandemic subsides, and we will be back to importing cheaply from China.
For us in the industry, it was a cruel joke to watch all kinds of hype being peddled in the name of Atmanirbhar Bharat. Even in a national calamity, many state governments indulged in large-scale Chinese imports, corruption and scandals in COVID-19 treatment; and Indian banks never lent to anyone on time. It was CDC Investment Works, a UK government development finance institution that came forward quickly to offer working capital, not Indian banks nor the state or central governments. We have payments as old as three years pending from the Andhra, Telangana and Chhattisgarh governments, and no amount of follow-up or legal notices help. The funny part is, these same governments are asking us to set up facilities in their states, promising crores in grants. This is the story of hundreds of companies working with the government. Unless this is fixed, we will never have an Atmanirbhar Bharat.
The only silver lining has been the speed at which the PMO and central ministries, Bharat Electronics Limited and DRDO worked to help us deliver the 30,000 units of Skanray ICU ventilators for which the Centre placed orders on BEL. It was a clean GoI to GoI transaction, with no scope for middlemen or graft.”














