Headlines of The Day
Holding 1.5% global market share, Indian MedTech sector to hit $20B

With the opening of 13 greenfield medical device manufacturing facilities, India is expected to establish itself as a leading exporter of medical devices.
In March 2024, the country’s Union Minister for Chemicals & Fertilizers and Health & Family Welfare, Mansukh Mandaviya, announced that under the Production-Linked Incentive (PLI) scheme, 26 applicants seeking to manufacture medical devices had been accepted for 138 products.
In a research report jointly published by KPMG and industry body APACMed’s, foreign direct investment (FDI) in the Indian medical device sector was US$3.4 million in the first three quarters of FY23, up from US$1.9 million in FY22 and the highest since FY17.
At present, India is among the world’s fastest-growing markets for medical devices, registering a growth of 2.89 percent in value shipments in 2022 over 2021, and growing at 6.35 percent CAGR in 2022, when compared to 2017.
In 2021, the domestic medical device market was estimated to be worth over US$8 billion. Analysts predict that by 2027, the value will have exceeded US$20 billion, growing at a rate of about 17 percent annually.
Increase in foreign investment
In March 2024, Medtronic, an international medical device company with its headquarters in Dublin, Ireland, revealed plans to invest US$350 million to develop its Medtronic Engineering & Innovation Center in Hyderabad, one of the biggest research and development facilities outside of the US.
The investment will support surgical and implantable technologies, robotics, imaging, and navigation. The multinational player in personal healthcare products, Omron Healthcare, with headquarters in Japan, said in May that it would establish a medical device manufacturing plant in Tamil Nadu for US$15.5 million.
Similarly, German MNC Siemens Healthineers also intends to invest US$197.7 million by 2025 to establish an innovation hub in India’s Bengaluru. In August 2023, Siemens and India’s Manipal Academy of Higher Education (MAHE) partnered for a master research collaboration in the medical industry. The center’s main areas of interest will include immersive technologies, cybersecurity, artificial intelligence, and data analytics.
Meanwhile, French diagnostics specialist multinational BioMérieux is also setting up a laboratory at Kasturba Medical College in Manipal.
Recent notable investments
In 2022, Warburg Pincus invested US$210 million into Meril Life Sciences, while Samara Capital contributed US$150 million to Sahajanand Medical Technologies, specializing in molecular diagnostics.
- Temasek Holdings and Oswal Alternates jointly invested US$85 million in Molbio Diagnostics, headquartered in Goa.
- Leapfrog Investments’ US$61 million in Redcliffe Genetics.
- Lightrock Venture’s US$32.8 million in BeatO.
- Sony Innovation Fund’s US$8.5 million in Tricog.
- Amicus Capital’s US$7.3 million in Rivaara Labs, focusing on molecular diagnostics.
After opening its first facility in Gurugram in 2016, Boston Scientific, a US-based company, launched its second R&D center in Pune in 2023. India is home to the company’s second-largest R&D centers outside of the United States of America.
Stryker, another leading player in the medical device industry, recently opened a neurovascular research facility at its Global Technology Center in Gurugram in an effort to find treatments for brain stroke.
National Single Window System launched to ease imports
To facilitate the efficient importation of medical devices, India has established a “National Single Window System” (NSWS).
Spearheaded by the Central Drugs Standard Control Organisation (CDSCO), the NSWS system was launched on January 1, 2024, with the aim of offering a consolidated platform for approvals and simplifying the import process for medical devices. The CDSCO is the regulatory agency that oversees the regulation and registration of medical devices.
India’s National Medical Devices Policy 2023
India’s National Medical Devices Policy 2023 (see Gazette notification here) was approved by the central government on April 26, 2023, with the objective of supporting the industry’s growth from US$11 billion in 2022 to US$50 billion in 2030. This ambitious goal implies at least 15 percent annual growth over seven years.
Under the National Medical Devices Policy 2023, there will be six major policy intervention areas: building enabling infrastructure, facilitating R&D and innovation, attracting investments, regulatory streamlining, human resource development, and brand positioning and awareness creation.
According to research reports, both global and Indian markets for medical equipment expanded by little more than 9 percent in 2022.
Industry leaders have reportedly welcomed the policy, which targets boosting India’s INR 900 billion (US$108.2 billion) medical device industry, currently ranked fourth in Asia behind China, Japan, and South Korea.
PLI Scheme for promoting domestic manufacturing of medical devices
India faces certain challenges in the medical device business, such as the absence of proper infrastructure, limitations in the local supply chain and logistics, high financing costs, insufficient supply of high-quality power, restricted design capabilities, and low investments in R&D.
To address this capacity deficit, the central government authorized the “PLI Scheme for Promoting Domestic Manufacturing of Medical Devices” on March 20, 2020, the guidelines for which were released in October the same year.
The PLI scheme, which is limited to greenfield projects, aims to increase local manufacturing and draw significant capital into the Indian medical device industry. The PLI program will run for fiscal years 2020–2021 through 2027–2028. Under the PLI scheme, selected companies (beneficiaries) will get financial incentives for a period of five years, at the rate of five percent of incremental sales of medical equipment manufactured in India. Thus, they need to meet stipulated production and sales targets to qualify for the incentives.
Eligibility threshold criteria for “Category A” applicant: PLI Scheme
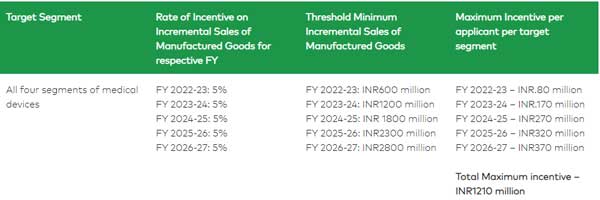
Eligibility threshold criteria for “Category B” applicant
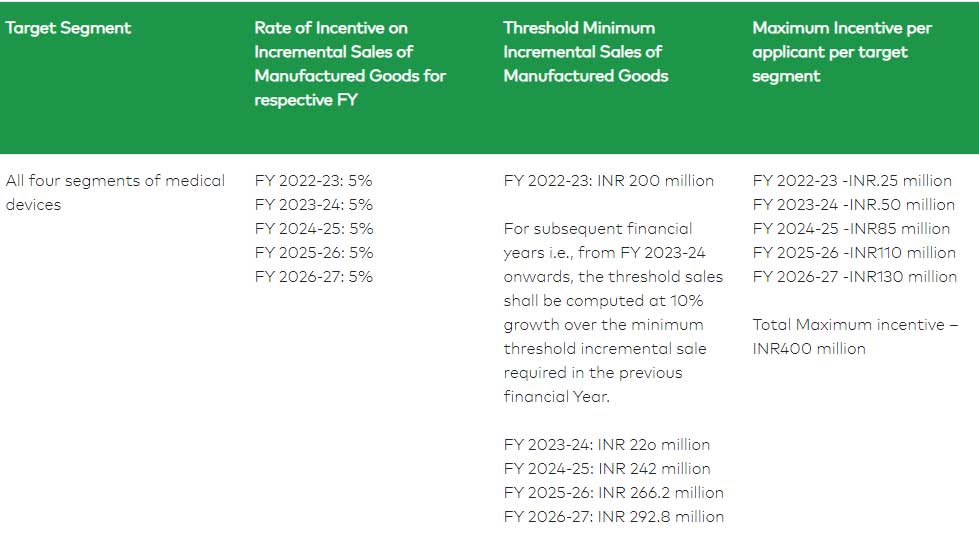
The products under the PLI scheme have been categorized under the following four targeted segments:
- Cancer care/Radiotherapy medical devices
- Radiology & Imaging medical devices (both ionizing & non-ionizing radiation products) and Nuclear Imaging devices
- Anaesthetics & Cardio-Respiratory medical devices including Catheters of Cardio Respiratory Category & Renal Care medical devices
- All implants including implantable electronic devices

For anyone manufacturing or importing medical equipment into India, obtaining a CDSCO Medical Device License is necessary. This license is granted by the state licensing authority, provided that the license holder satisfies all conditions outlined in the Medical Devices Rules. Furthermore, the authorities covered by the Central Licensing and Approving Authority (CLAA) system must be notified before any medical devices can be manufactured.
Emergence of MedTech parks in India
With a cap of INR 1 billion (approx. US$12 million) per park or 70 percent of the project cost of shared infrastructural amenities, whichever is lower, the Promotion of Medical Device Parks initiative offers grant-in-aid to medical device parks.
Financial support for shared infrastructure facilities has been approved by the central government for four medical device parks located in Himachal Pradesh, Tamil Nadu, Madhya Pradesh, and Uttar Pradesh. It is anticipated that the parks will be completed by June 2024.
The purpose of the ‘Promotion of Medical Device Parks’ initiative is to establish top-notch “Common Infrastructure Facilities” in medical device parks, thereby facilitating convenient access to standard testing and laboratory facilities across the country.
Such dedicated spaces for the MedTech industry are expected to support the development of a strong ecosystem for domestic medical device manufacturing by lowering production costs, boosting competitiveness, and thereby ensuring affordable and better accessible medical equipment in the market.
- In March 2024, it was reported that five firms had expressed their intention to invest a total of INR 1760 million (US$211.4 million) in the Madhya Pradesh Industrial Development Corporation’s (MPIDC) Medical Devices Park in Ujjain. The MPIDC will grant roughly 26 acres to create a dedicated space.
- Official data from the MPIDC revealed that Medqverse Private Ltd., one of the five firms, intends to invest INR 1 billion (US$12.02 million) to set up a unit for radiation cancer treatment equipment, which is estimated to provide job opportunities for 300 people.
- Meanwhile, the Andhra Pradesh Medtech Zone Ltd. (AMTZ) project has received financial support worth INR 250 million (US$3 million) from the Department of Pharmaceuticals in 2022. Its funding was granted under the sub-scheme “Assistance to Medical Device Industry for Common Facility Center” for the construction of a common facilities center for the testing and study of superconducting magnetic coils. Overall, the zone has received INR 2249 million (US$27 million) from the Department of Pharmaceuticals.
- The state of Uttar Pradesh is also attempting to establish itself as the industry leader in the fields of pharmaceuticals and medical devices. The chief minister of the state dedicated the 2000-acre projected Pharma Park in March 2024 with the goal of establishing Uttar Pradesh as a center for pharmaceutical production and export.
Outlook
The policy to promote the establishment of medical device parks is expected to improve India’s capacity to produce essential medical equipment. In addition to drawing large investment, the move is anticipated to provide plenty of job possibilities for the local populace.
India may also consider using the public-private partnership model to grow its market for medical devices and create synergies in fields where it lags capacity, technology know-how, and infrastructure.
The European Commission and private consortiums, notably MedTech Europe, launched the Innovative Health Initiative (IHI) in 2021 to foster cross-sectoral innovation in biotechnology, digital health, and medical technology. PPPs have the potential to stimulate innovation in financially challenging situations with intense cost constraints. India Briefing












