Industry
LIS – Cutting-edge technology meets precision and accuracy

Never before have we seen such a revolution in data management as with laboratory information systems (LIS).
Advanced computer-based platforms are designed to streamline every aspect of laboratory operations, from sample tracking and processing to data analysis and storage. Driven by rising demand for lab automation and advancement in the R&D labs, especially in the pharmaceutical and biotechnological laboratories, the high accuracy and efficiency results of LIS are driving growth.
The market
The global laboratory information system market, also referred to as LIMS (laboratory information management system) is expected to reach USD 3.5 billion by 2030, expanding at 6.7 percent CAGR from 2023 to 2030.
The need for efficient management of laboratory data and the increasing adoption of electronic health records (EHRs) are the key drivers. The Indian government’s focus on improving healthcare infrastructure and providing access to quality care for all citizens will also push demand.
The rise in the number of life-threatening diseases, and prevalence of non-communicable diseases, has resulted in the need for quality samples. Moreover, the rise in the number of genome and DNA studies, and in vitro ADME technologies, are some of the factors propelling the growth of this market. Additionally, the growing R&D applications, especially in the pharmaceutical industry, such as generic drug development and genome and cancer studies, are anticipated to fuel market growth in the coming years.
Lab automation reduces human errors while performing repetitive tasks, such as pipetting and moving plates. It also improves accuracy. Lab technicians need not be concerned about oversights. The technological advances in recent years have resulted in an increased demand for laboratory data to meet microbiological, clinical, and public health needs.
The installation of LIMS in many areas as student health centers, hospitals, provider groups and clinics, universities, pathology laboratories, public health, independent reference laboratories, veterinary laboratories, and toxicology clinics is adding to its populatity.
One of the biggest benefits of LIMS software is having a centralized system to manage all the peripheral components of a lab utilized in executing a workflow. (This is a benefit whether you are using a LIMS system for small laboratories or enterprise-size facilities.) Information about studies, instrumentation, and inventory can be stored in one centralized location, easily accessible to the lab team when needed.
Using LIMS for study management. Lab samples are typically collected in support of a higher-level research study or project. LIMS provides organizational structure to group-related samples together, underneath the appropriate study or project. These studies typically have subjects associated with them (human or otherwise), which can be grouped together into cohorts with associated visits. A fully functional LIMS software allows researchers to manage all this information in a single system, thus centralizing all the information for more robust data management.
Using LIMS for inventory management. Integral to any lab’s ongoing operations is its reliance on lab supplies, such as reagents and chemical inventory. To execute protocols effectively, a laboratory’s staff must have the necessary inventory on hand. LIMS is an important tool to provide the lab with insight into what material is on hand and what might need to be ordered. As personnel uses supplies, LIMS can automatically mark the supplies as consumed and trigger a re-order process. This limits the potential for error or delay, and improves a team’s efficiency. LIMS also provides lot number tracking, so the specific reagent used to process a lab sample is recorded in the system for future audit purposes.
Using LIMS for instrumentation tracking. Every lab has a variety of instrumentation it uses on a day-to-day basis, including liquid handlers, QC instrumentation, and sequencers. It is critical for a lab to track the maintenance and calibration these instruments use, so the test data they generate is deemed accurate. LIMS tracks all this activity and automatically alerts users when maintenance is pending or overdue. It also can track any issues that arise with lab instrumentation as they come up.
In addition to these key features, LIMS software provides the reporting, analytics, and collaboration functionality to make all the data that is captured above useful. From a reporting standpoint, LIMS can provide information on any aspect described above. Example reports include protocol duration, sample throughput, reagent expiration, and instrument uptime. Dashboards can be set up to provide high-level trending. For example, dashboards could be created to display the number of submissions over time, lab staff utilization, and sample status.
Thirdwave analytics
Based on the end-use segment, the life sciences industry was the largest, accounting for a share of over 40 percent in 2022. Rapid advancements in healthcare due to rising R&D are anticipated to fuel the demand for LIMS during the forecast period
Based on the component, the services segment accounted for the largest revenue share in 2022 owing to rising laboratory automation
North America accounted for the largest market share of over 44 percent in 2022. The presence of well-established pharmaceutical companies and research laboratories, and higher awareness about LIMS among end-users, are expected to drive its demand in the region. An increased demand is emanating from enhanced clinical research in India, China, Brazil, Singapore, Malaysia, and Middle-Eastern countries.
Vendors
The top four players of global LIMS labware, Thermo Fisher Scientific, LabVantage Solutions, and Starlims Corporation dominate with a combined 80-percent market share.
Siemens; PerkinElmer Inc.; Autoscribe Informatics; Illumina, Inc.; Labworks; LabLynx, Inc.; Computing Solutions, Inc.; CloudLIMS.com; Ovation; LABTRACK; and AssayNet; CliniSys Group Allscripts Healthcare LLC.; Orchard Software Corporation; CompuGroup Medical AG, Prolis, Medical Information Technology Inc.; and Epic Systems Corporation are also aggressive.
The LIMS industry is competitive with the presence of so many companies. These involve implementing strategic initiatives, including acquisitions, collaborations and partnerships, and new product launches. Vendors are constantly upgrading their existing LIMS solutions to sustain their market share. Starlims Corporation, formerly Abbott Informatics, has launched LIMS for mobile phones so that data can be accessed anytime, irrespective of location. Besides, it released the Starlims technology platform V12.2. The release will include a modernized electronic lab notebook system, using innovative HTML5 technology.
In September 2022, Thermo Fisher Scientific, Inc. released version 7.7.1 of Watson Laboratory Information Management System software, designed to enable the hosting of Watson in a cloud setting, while satisfying the requirements of 21 CFR Part 11 – Open System. And Illumina released LIMS v7.0, enabling the deployment and running of the Illumina LIMS software-provided hardware, including virtual machines (VMs).
Increasing adoption of LIMS Market is contributing to the growth of the hops-extract market through 2030. The demand for hops extract is increasing continuously and the complex process of extraction increases turnaround time for hops extract. Adaptation of new technology will provide thrust to the production process of hops extract, and help to fulfil the increasing demand for hops extract in 2023 and beyond.
Core components of LIMS
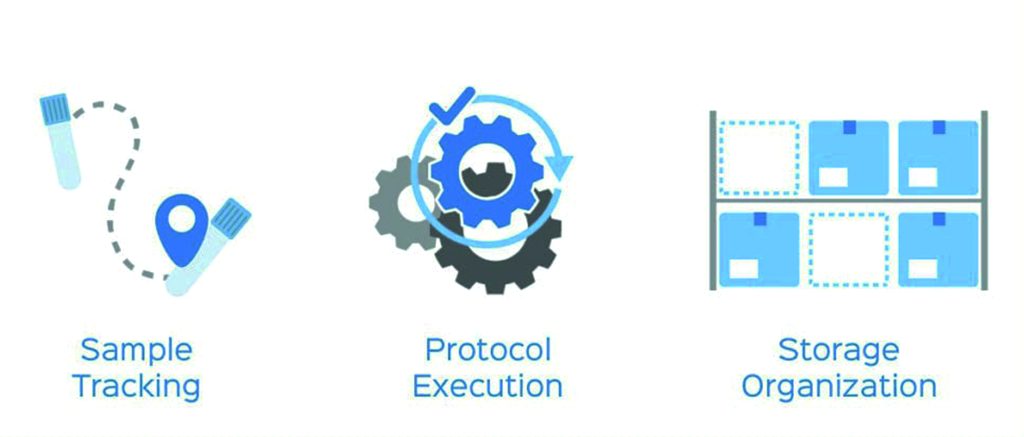
A good LIMS has three main components – sample tracking, protocol execution, and storage organization. Imagine a lab in which samples are tracked differently by different researchers, using methods varying between a pen and paper and a massive spreadsheet. It would be extremely difficult to ensure the data is not compromised by human error – missing results, errors, and differences in data collected can compound any mistake.
Thirdwave analytics
Sample tracking. The primary function of LIMS is to track a sample from the time it arrives in a laboratory through its testing and storage. This includes recording all data associated with the sample upon its initial accession, such as the sample’s ID, source, collection date, and quantitation information (i.e., concentration, volume, and particulate amount). As the lab sample progresses along its workflow, additional data is captured, which is also stored in the LIMS. This includes test results, derived sample data, and time-based study metrics.
In addition to capturing and tracking data specific to each lab sample, LIMS also tracks who has interacted with the sample and where the sample was throughout its lifecycle. For example, a sample may be placed in a batch or pooled together with other samples the laboratory is testing. This means the lab sample must be tracked not only by an external barcode label affixed to the test tube but also by a chemical index. This allows it to be identified from the other samples in the pool. All of this valuable sample-tracking information is stored in the LIMS system.
LIMS software is also critical for developing a lab’s workflows and protocols.
Protocol execution with LIMS
The second major function of LIMS software is to drive the standardization of a lab’s workflows and underlying protocols, procedures, and steps. Ensuring that each lab tech adheres to the specific steps in a published SOP (standard operating procedure) when processing a sample, regardless of who is processing the sample or running a test, is critical to obtaining an accurate and repeatable result. LIMS supports standardization across the laboratory team by digitizing the steps in procedures and protocols. This ensures the entire lab staff executes the correct steps, in the correct order, when running a sample through a test.
LIMS software can manage test assignments so that when a sample arrives in a laboratory, it is immediately assigned the appropriate protocol. LIMS can also provide a lab with more stringent protocol version control measures, granting visibility to research or clinical teams, depending on who is authorized to run the protocol. Lab test results can be recorded, sent through the appropriate approval queue, and then distributed to the necessary team members via reports
Storage organization with LIMS
The third critical feature in LIMS is keeping track of where a sample is throughout its laboratory lifecycle. Starting with the individual lab sample, the LIMS tracks where, in a particular box, the sample tube or vial is kept (for example, slot A1 or B5). Next, the system keeps track of which drawer that box is in, and which rack the drawer is in. Furthermore, the system tracks which shelf the rack is on, and which room the freezer is in.
This storage hierarchy (Sample > Position > Box > Drawer > Rack > Shelf > Freezer > Room) plays a critical role in locating samples quickly in busy laboratories. Research teams that know exactly where lab samples are stay productive, organized, and efficient.
Three options
Consumers have the option of choosing between on-premise, cloud-based, or remotely hosted LIMS. Each of these solutions caters to the various needs in the market.
On-premise LIMS requires the laboratory to own the proper infrastructure to host it – you need compatible hardware physically on-site to run it. The company has complete control over the system, which includes doing regular maintenance on the database and servers and ensuring data security. It also takes much longer for on-premises LIMS, as it requires equipment purchases, installation, validation, etc.
As opposed to the on-premises LIMS, a cloud-based system is hosted outside the organization. All the responsibility for installing, updating, and managing the software is on the vendor who provides it. The supplier provides support and maintenance, making it easier for smaller laboratories that do not have their own well-trained IT departments. There is no need to manage the software locally, allowing the lab team to focus on more important tasks while maintaining smooth laboratory workflows.
Another distinguishing factor is the method of payment. On-premises LIMS requires a full, one-time fee, whereas cloud-based services are subscription-based, requiring smaller monthly payments instead of a large one.
The issue of data storage is easily solved in cloud-based systems, as even large amounts of laboratory data of various formats and sizes can be stored in this way. Data backups may be performed automatically, and cloud storage, when correctly configured, is just as secure, or even more, as a traditional local server.
The cloud-based product segment dominated the market in 2022, and is anticipated to expand further at the fastest CAGR over the next five years. With its heightened security, options for management of daily processes and maintenance, reduced strain on internal IT, and range of customization options and validation extents, a cloud-based LIMS solution is a smooth fit for most laboratories.
While a cloud-based system appears to be a good idea for laboratories across many industries, it is not without flaws. There are some significant drawbacks to be aware of:
Data transfer times. Cloud-based LIMS technology allows laboratories to upload their data to a server instead of keeping it physically in the labs. While it can help the facility go digital and streamline its workflow, there are drawbacks too. Uploading and downloading data takes longer than accessing files on-premises, and can consecutively slow down the lab operations, but we need to remember that this affects only the big files, which is mostly not a standard for daily work.
Data protection concerns. Files are stored outside the laboratory, on remote servers. This can raise concerns about data safety, as it might be more prone to hacker attacks and data leaks. Information security is of utmost importance nowadays, so it is crucial to check what security measures the software provider offers. We need to remember that there are more security specialists working for big providers than in the internal company teams.
Connection requirement. Since the cloud-based LIMS software is accessed via a web user interface, it will not work without reliable and fast internet. This means it might not be suitable for laboratories in locations lacking a good internet connection, and in case of connectivity issues, the system will not be able to operate.
Possible cloud downtime. Like any technology, cloud-based systems have their share of imperfections. The cloud could become inaccessible suddenly due to a power outage in the provider’s server room, and it may become unavailable due to a scheduled reboot or maintenance. It can have an impact on laboratory operations, and lab managers have no control over it.
Overall, the choice of LIMS for the laboratory is determined by the budget, payment preferences, and the size of the laboratory. Small startups have fewer needs – and fewer funds – than big facilities. While both types can benefit from the cloud-based lab information management system, there are different issues to consider for individual laboratories.
It is undeniably worth it if financial resources are limited and the lab cannot afford to pay upfront for the implementation of a regular LIMS. A cloud-based system is also advantageous if there is the need for a system that can be quickly set up rather than waiting months for everything to be up and running.
A properly set up cloud-based LIMS is as secure as an on-premises one, and it complies with all standards and requirements as well.
The operational expenses, which include expenditures associated with the software licencing, training of the healthcare workforce, maintenance costs, and service charges, are one of the significant factors restraining the growth of the LIS market. The cost of maintaining IT systems is more than the actual cost of the program, making it challenging for many small and medium-sized laboratories to invest in LIS.
The cost of training and implementation accounts for around 15 percent of the total cost. This system needed skilled personnel, who could handle everyday tasks and manage patient data with effective security to prevent the abuse of personal and medical information. One of the main things impeding the growth of the LIS industry is security concerns.
Outlook
Every medical laboratory needs a reliable, stable information system to function effectively. The industry is going through an exciting period right now, when technology has advanced to the point that one can assist their clients in building a linked environment that speeds up science and boosts laboratory productivity.












