Industry
MedTech companies must move faster on Generative AI

To realize the promise and potential for GenAI, MedTech executives must make swift and targeted efforts. BCG’s 6P framework can help leaders understand the full scope of the undertaking and focus on the top priorities as they pursue the MedTech GenAI imperative.
The 19th century British Prime Minister George Canning observed, “Indecision and delays are the parents of failure.” His insight, offered as sailing ships were giving way to steamers, carries even greater currency two centuries on as technology changes our lives at a speed unimaginable to our forebears. Nowhere is this more evident than in the burgeoning field of generative artificial intelligence (GenAI).
As noted in BCG’s recent publication, “MedTech’s Generative AI Opportunity,” the technology promises to boost efficiency, enable greater personalization of care, enhance product and service innovation, and uncover exciting new insights from the bountiful data generated daily by an ever-expanding fleet of digitally enhanced devices and equipment. The imperative for MedTech companies, therefore, is to get started.
Create Value–and Do No Harm
MedTech executives are right to want to separate the hype from reality and understand the risks. They have concerns about data security and privacy, and they want to instill sensible guardrails, establish accountability, and have practical ways to pull GenAI solutions out of service if they misfire. Add to these issues the question of how regulatory and legal frameworks will evolve, and some business leaders might lean toward a wait-and-see approach. But others who have a head start in basic AI infrastructure may find the opportunity to seize a GenAI first-mover advantage too alluring to forego. Leaders who dawdle might never catch up.
Indeed, MedTech players from Alcon to Zimmer Biomet have all announced their efforts in the exciting new field. While clinical use cases are still relatively rare, 3M Health Information Systems has recently shared plans to integrate generative AI into its conversational AI platform to improve physician workflow experience. Its model seeks to merge electronic health record data with patient-physician conversations to generate post-encounter medical notes ready for physician review and sign off.
Meanwhile, the market is awash with GenAI technology startups claiming to be the next unicorn and commanding commensurate valuations. Most will not live up to their launch price, nor would they even be fit for purpose for a MedTech acquirer. For MedTech leaders, therefore, preparing your GenAI investment thesis requires a firm understanding of where your company will employ GenAI and a balanced view of the upside and downside implications of putting the technology to work. These channel markers set the parameters for in-house investment and partnerships—whether these initiatives are transformative, capability building, or tuck-ins providing functional efficiencies.
Getting to Scale in GenAI–Quickly
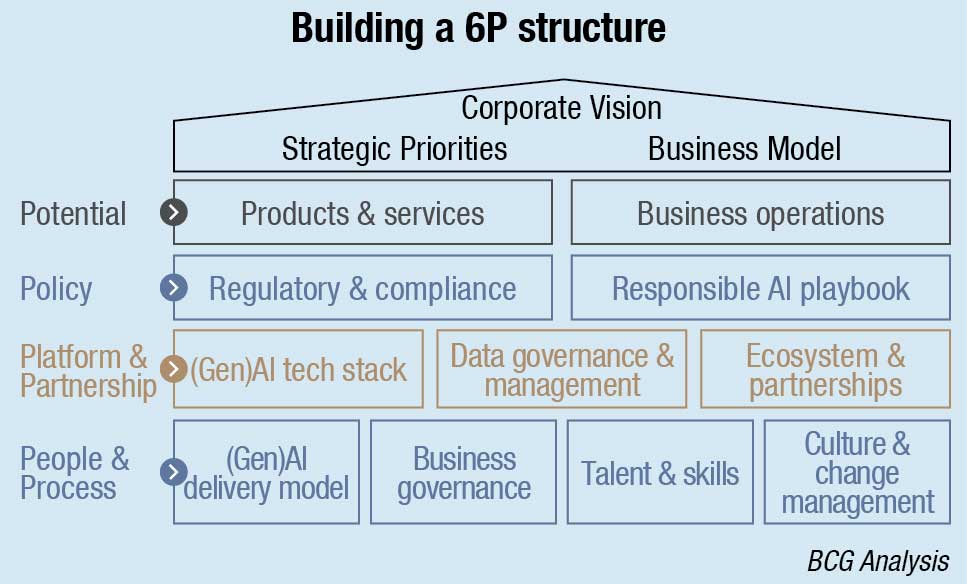
A common misconception holds that launching GenAI solutions is a mostly technical endeavor. BCG’s experience suggests that less than half of the implementation effort involves the technical platform, including building the model, securing and structuring the data, and setting up the required infrastructure. Rather, the bulk of designing and managing GenAI solutions and products requires picking the right technology partners, creating thoughtful and responsible corporate policy, and developing a business structure, HR system, and core processes that can adapt with the potential that GenAI brings. Getting these aspects of a GenAI transformation right is critical if MedTech GenAI solutions are to fulfill their promise.
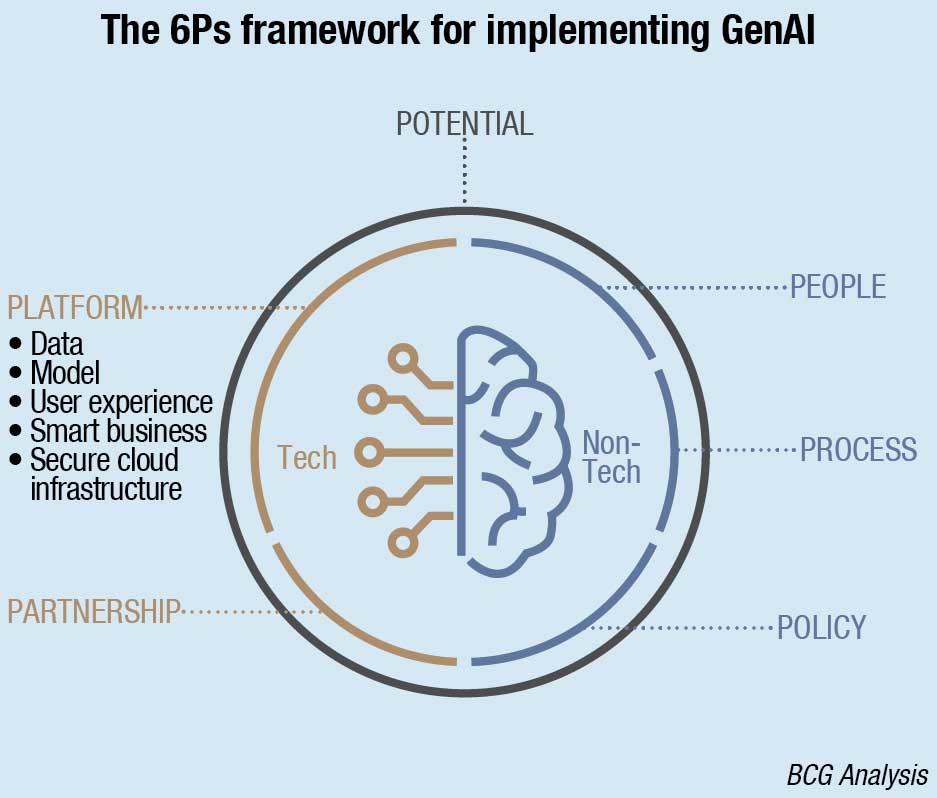
To help MedTech companies launch and manage their GenAI solutions, BCG has developed the 6P Framework. Some of these guidelines, such as Policy, Platform, and Partnership require extra caution in the MedTech space given the ethical concerns in healthcare and the sensitivity of patient data. Others, such as People and Process are less industry specific, but the change management aspect of GenAI adoption will be no less challenging in MedTech than it is in other industries. The six elements of the framework can be described as follows:
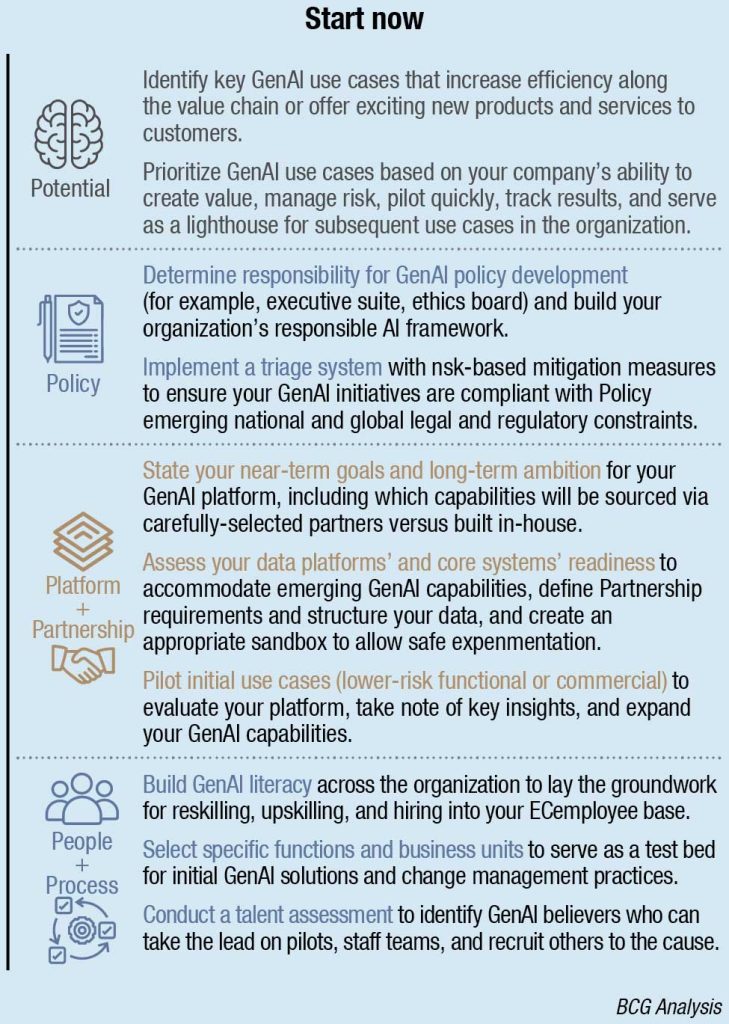
Potential
GenAI is uniquely suited to tasks that incorporate a high volume of unstructured data inputs and generate coherent output in the form of text, code, images, and video. GenAI can relieve the burden of tasks that are highly repetitive but not fully automated because of workflow constraints. GenAI can address common business frictions and pain points, such as sales support, bionic service agents, simulation training, and document processing, not to mention factory floor improvements. By upgrading operating efficiency and enhancing both the customer’s and employee’s experience, GenAI can drive business growth and margin improvement. Rather than trying to launch a GenAI-enabled clinical decision support product out of the gates, newcomers to the field may wish to start with lower risk functional or commercial GenAI applications, such as customer service, and then iterate quickly to gain valuable experience and confidence.
Policy
MedTech companies maintain a fiduciary duty to drive value for shareholders while safely enhancing the well-being of patients and providers. A core element of the Policy effort, therefore, involves building a responsible GenAI framework that covers enterprise-wide strategy, governance, key processes, technology and tools, and culture. MedTech leaders need to ensure clear data ownership, privacy, carefully monitored scope, and safe, regulatory-compliant AI product development cycles. Installing responsible GenAI guardrails allows employees to safely experiment with potential GenAI opportunities and use cases, so MedTech companies can uncover pitfalls, identify tradeoffs, and learn to mitigate risks early.
For example, GenAI can sometimes deliver content that is flawed or, more perniciously, plausible on the surface but objectively incorrect (so-called “hallucination”). GenAI sometimes inexplicably neglects to give vital information (“omittance”). Additionally, content generated from unlicensed or proprietary source data may lead to copyright infringement or data privacy infractions. These risks are particularly acute in foundation models that will deal with patient data or develop clinical recommendations, so MedTech companies must put in place processes and policies to mitigate bias in data sets and prompted outputs. A fully evolved corporate policy for GenAI will communicate the right tone and culture from the top, create awareness and build a code of conduct, establish whistleblowing processes, and define roles and responsibilities for ensuring compliance within your company and its ecosystem of partners.
Platform and Partnership
A MedTech GenAI platform is more than just a model. It is an end-to-end stack consisting of five primary layers––data, model, user experience, smart business, and secure cloud infrastructure. With GenAI, MedTech companies must navigate both model and data complexities, such as how to select and train models on regulated data, how to manage access, and how to validate model output. This may involve off-the-shelf public models or taking a public model in-house for more extensive customization (called a “managed model”). MedTech companies generate many data streams, but they will need to curate their proprietary inputs to feed and train models in a secure way. MedTech GenAI solutions will require robust technology to manage source data, classify and tag sources to quantify risk, enable faster adaptation to regulation, and increase speed in experimentation and delivery. Healthcare professionals will want to understand and trust the reasoning behind AI-generated solutions before they will use such services to make clinical decisions. Techniques such as attention maps or saliency analysis can help MedTech companies push forward, but your overall GenAI platform must use secure design techniques, strong cybersecurity capabilities, and have in place specific guidelines for identifying and mitigating security risks unique to GenAI.
Even the largest and most capable MedTech players will need to consider partner/build/buy decisions as they set up their GenAI platform. Selection criteria will be complex and variable both for platform partners (for example, geography, portability, security, and privacy) as well as model partners (such as model size and output, fine-tuning, and compatibility with the selected platform). For common operational issues such as functional solutions, off-the-shelf offerings will provide a cost-effective avenue to notch up early gains. However, as companies move into the specific requirements of industry participants and core capabilities that determine competitive advantage, they will need to be more circumspect about partner selection and exclusivity, their chosen partner’s ability to predict and mitigate risks, access to different foundation models, and a host of operational and cost/benefit considerations.
Rarely has any discipline evolved as fast as the GenAI space. A partner that is strong today may become irrelevant in months. MedTech companies should monitor the evolution of their potential partner ecosystem, bringing in new partners and capabilities as required. MedTech companies may need a neutral business advisor to cut through the fog and integrate the disparate pieces so they can start their GenAI journey.
People and Process
The most important people decision in GenAI is to establish ownership of the topic at the CEO and top executive level, setting clear goals and guidelines for how and why your MedTech company intends to use GenAI. At the outset of the GenAI journey, MedTech players may be able to introduce their innovative solutions without reconfiguring the organization. However, as GenAI solutions take hold, company leadership should address fundamental questions about operating models, including the composition of use-case design and implementation teams, how GenAI tools will affect job functions dedicated to core processes, and where new GenAI talent will report. Building a talent pool that can identify, develop, launch, and maintain GenAI solutions will necessitate training and upskilling, as well as a broad-based change management program to adapt to the new reality. These questions are likely to affect distinct parts of the organization at different speeds, as lower-risk functional use cases (for example, accelerated accounts-receivable system, optimized indirect marketing spend) may well be rolled out before high-stakes solutions that directly impact individual patient outcomes.
Getting in the Game––Safely
At this early stage in the evolution of GenAI in MedTech, the imperative is to get started. The 6Ps we have just described represent work streams that your company should initiate.
Within that structural framework of the 6Ps, there are ten checklist items that your organization should be doing right now to get in the game:
Deploying GenAI and adhering to the Hippocratic oath to “do no harm” represents a challenge in the MedTech industry, where regulatory lanes are not fully marked and the stakes for patients can be a matter of life or death. But MedTech companies sizing up the GenAI opportunity have excellent reasons to be excited about the potential for solutions across the entire value chain. Bearing in mind a timely observation from British Prime Minister Canning two centuries ago, companies must cast aside the indecisions and delays––which serve “the parents of failure”––overcome their inhibitions, and embark on their GenAI journey. With a responsible GenAI policy in place and a road map on how to get in the game safely, your MedTech organization can help transform how healthcare is conceived and received in the decades to come.
The authors would like to thank David Tang-Quan and the following for their contributions to this article: Vikram Aggarwal, Nadya Bartol, Ian Chen, Marjolein Cuellar, Torben Danger, Meghna Eichelberger, Michael Farrugia, Stefan Leve, Raph Mannino, Shekhar Marathe, Dan Martines, Tom Retelewski, Barry Rosenberg, Kirsten Rulf, Michelle Russell, Steffen Simon, Stephen Spencer, Krishna Srikumar, and Helen Zuo.
Courtesy: Boston Consulting Group












