Mass Spectrometers
MS, Helping Research Attain An Even Greater Vantage Point Than Before
While MS-based techniques will continue to grow, its growth trajectory with certainty cannot be predicted. With MS-based techniques merging with next-gen DNA sequencing, automation replacing other chemical and immunological analysers, with breakthroughs in miniaturisation MS serving as POC testing devices , any number of possibilities can emerge.
The mass spectrometer is now a sensitive, specific, and versatile analytical tool in the clinical laboratory that has recently undergone rapid development. The biochemical researchers and clinical scientists are relatively late adopters of the technique. In fact, when mass spectrometry (MS) was first adopted by clinical laboratorians in the early 1970s, it was only an investigative tool for metabolic profiling in urine and other body fluids. With the rapid development of immunoassays in the 1970s to 1980s, first with radioactive labeling, then with enzymes and fluorophores; and the paradigm shift from phenotyping toward genotyping in the 1990s, largely facilitated by PCR, automated DNA sequencing technologies and human and microbial genome projects, the many virtues of MS-based techniques were outshone. Coupled to the often prohibitive instrument cost, requirement for interpretative expertise and lack of substantial automation in many MS-based techniques, the application of MS in the clinical laboratory remained limited and, with due respect, often regarded as a reference or verification technique, for drugs, hormones, and one of the chemotaxonomic techniques for establishing microbial phylogeny.
While many of these applications remain pertinent to today’s clinical laboratory, pathologists have also been witnessing a disruptive change that is being brought about by MS-based technologies. Manual or semi-automatic phenotyping of microorganisms is being seen to vanish into the literal dark and MS-based microbial identification emerges as literal identities with quality scores from computer screens. MS is fast becoming an indispensable field for medical professionals. The mass spectrometers are being applied in various areas of medical diagnostics. From initial use in metabolic profiling, they have matured into applications including clinical toxicology assays, target hormone and metabolite quantitation, and more recently, rapid microbial identification and antimicrobial resistance detection. The mass spectrometric analysis of metabolites and proteins promises to revolutionize medical research and clinical diagnostics. As this technology rapidly enters the medical field, practicing professionals need to take full advantage of its capabilities.
Technology developments in MS have given rise to several applications of structural biology, both at the single protein and protein complex level. With the advent of new reagents, methods, and software, it is now possible to combine MS in unprecedented ways with traditional approaches such as protein crosslinking, affinity purification, limited proteolysis, and hydrogen-deuterium exchange. While the traditional coupling of MS with liquid chromatography (LC) and gas chromatography (GC) continues to be a robust method, technological advances have seen the rise of other new technologies, such as matrix-assisted laser desorption (MALDI) being directly coupled with MS, making it adept for even more applications. For example, the latest MS technologies can simultaneously analyze many features in one cell, which could help researchers discover novel targets with the potential for translation into new therapies. Computer technology and structural biology have played important roles in the maturation of this field. MS-based technologies have the potential to replace current analytical technologies, and existing expertise and instrument will undergo rapid evolution. Significant automation and adaptation to regulatory requirements are underway. Mass spectrometry is unleashing its potentials in clinical laboratories.
A number of instrument platforms have recently entered the clinical space, with either Class 1 Medical Device or FDA-approved IVD status. There are, however, significant roadblocks to gaining greater traction on the complex clinical terrain. Notable advancements promise to increase the quality, depth, and speed of proteomic data generation. These, in turn, may support higher levels of achievement for research and clinical MS applications. Recent technological advancements in high-resolution MS have helped create a clearer and more detailed picture of biological processes. Through the enhanced elucidation of biomolecular structural information and characterization of cellular networks, MS provides more information than ever before. With the rapid growth in the volume and complexity of MS data, accurate and reproducible analysis can be challenging. The ability to acquire, analyze, manage and report data generated in MS systems is evolving as new applications are discovered and current uses improved. Innovations in MS software address specific applications for in-depth analysis as well as provide improved functionality and compatibility for broader use and easier data access and management.
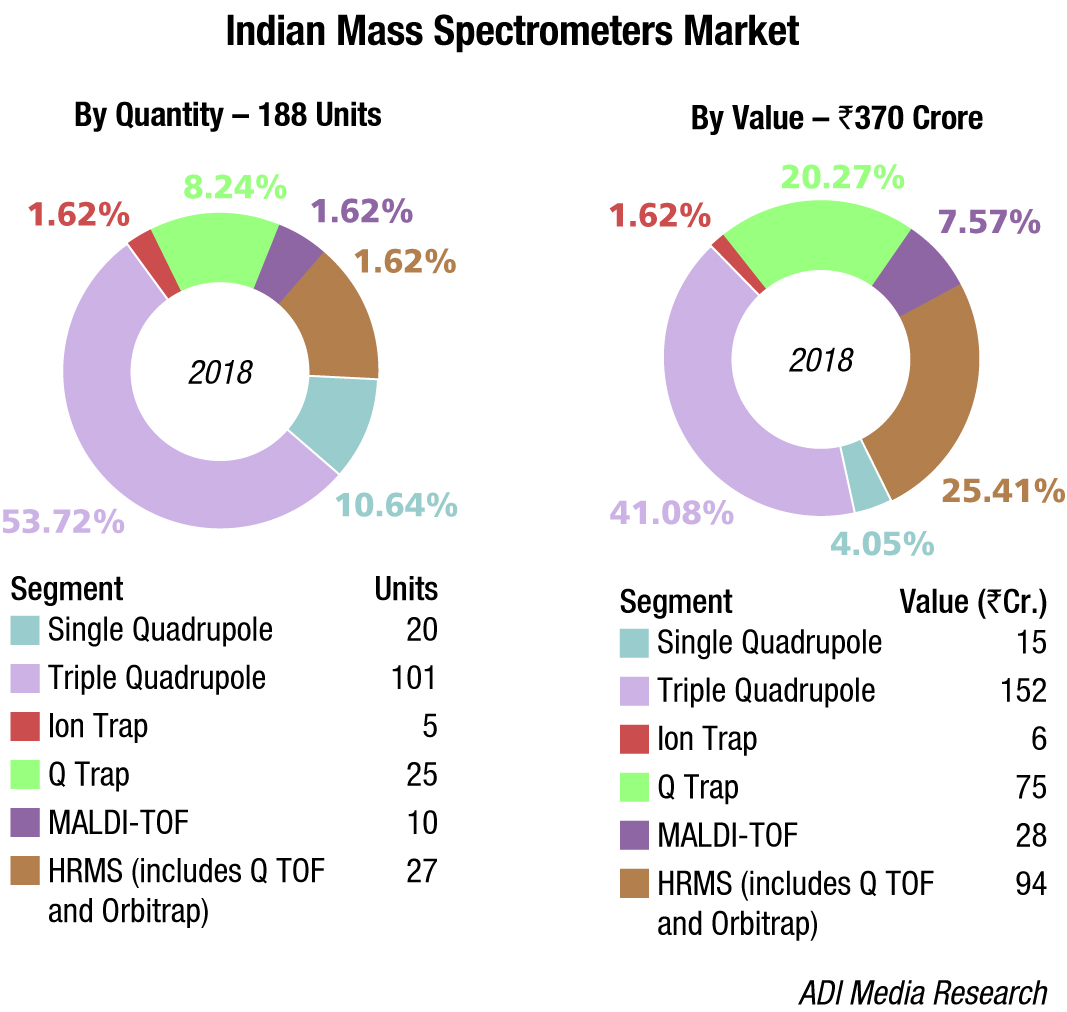
Indian market
The Indian mass spectrometers market is estimated at Rs 295 crore, a 10 percent increase over the 2017 market of Rs 268 crore. In 2018 estimates, we have also included Q trap, catered solely by Sciex, thus taking the total market to Rs 370 crore. The triple quadrupole segment saw the fastest growth and dominated the market with 41 percent share, by value and 54 percent share, by volume, driven largely by the pharmaceuticals and food safety segment.
HRMS, including Q TOF and Orbitrap, catered to by almost all the vendors, including SCIEX, Waters, Shimadzu, Agilent, and Thermo Fisher gained traction in 2018. Sales of ion trap, marketed solely by Thermo Fisher remained stagnant. The single quadrupole market continues to have a presence in the Indian market.
The market is forecast to grow at a CAGR of 20 percent over the next 4 years due to the increasing use of MS as the primary detection tool in the pharmaceutical industry for drug discovery pipeline and for quality assurance/quality control purposes. The Indian government, academia, R&D institutes, life sciences, and pharmaceutical industry are the biggest buyers of MS instruments. There is a steady increase in the number of contract research organizations in India, especially in clinical research management. With further technological advancements in instrumentation and software platforms, the R&D sector will grow. With GDP growth, these sectors are expected to pick up, causing increased demand for the MS instruments.
Many companies are constructing their manufacturing and research facilities in India. In addition, an increasing number of conferences and exhibitions by manufacturing companies to promote mass spectrometry technology is also contributing to the growth of the market.
Multinational companies such as SCIEX, Shimadzu, Waters, Agilent, and Thermo Fisher Scientific dominate the Indian market. Indian companies are still lagging in terms of the technical expertise required for manufacturing these high-end devices. Instrument manufacturers are continuously focusing on new product design and development that are compact, multi-disciplined, offer high accuracy, and are low cost. Market leaders depend heavily on their channel partners for the sales and distribution of their instruments. The instruments are manufactured out of India and imported to India; however, manufacturers are themselves involved in creating brand awareness and marketing their products through Indian operations.
Indian equipment manufacturers provide cost-effective products, but the quality and standards are not at par with the global market leaders operating in the Indian market. However, this trend is changing and with sufficient government push, R&D facilities, and infrastructure, the Indian companies can create a brand name and a niche in the market. Lack of skilled workforce is another challenge that Indian instrument manufacturers face. Manufacturers not only need to keep in mind that their products need to be world-class but also need to cater to and address the Indian customers’ requirements and challenges. Such an innovative product design and cutting-edge technology would allow instruments manufacturers to grow in the market that offers immense growth opportunities in the medium and long term.
| Tier I | Tier II | Tier III | Others |
|---|---|---|---|
| Sciex Shimadzu | Waters, and Agilent | Thermo Fisher | Bruker and Perkin Elmer |
| *Vendors are placed in different tiers on the basis of their sales contribution to overall revenues of the Indian mass spectrometers market. | |||
| Segment | Major Players |
|---|---|
| Single Quadrupole | Shimadzu, Waters, and Agilent |
| Triple Quadrupole | Sciex, Waters, Shimadzu, and Agilent |
| Ion Trap | Thermo Fisher |
| Q Trap | SCIEX |
| MALDI -TOF & Maldi Top TOF | Bruker and Shimadzu |
| HRMS (includes Q TOF and Orbitrap) | Sciex, Waters, Shimadzu, Agilent, and Thermo Fisher |
| ADI Media Research | |
Global market
According to Transparency Market Research, the global mass spectrometers market was valued at USD 5896.3 million in 2018 and is expected to reach around USD 10,884.8 million by 2026, expanding at a CAGR of 7.7 percent from 2019-2026, as the market will continue to be influenced by a range of macroeconomic and industry-specific factors. Growth in pharmaceutical and life science markets, rise in demand for clinical analysis, increase in mergers and acquisitions, and increase in food and beverage testing has influenced the penetration and growth of the mass spectrometer market globally. Technological advancements in MS have led to increased applications of this procedure. In addition, the rise in private funding and government grants for research and development activities of mass spectrometers supplements the market growth. On the other hand, high-cost mass spectrometers is the glaring restraint over the market for the same. In addition to that, regulatory policies and unfavorable political and economic conditions are also expected to negatively impact the mass spectrometer market in the near future.
Currently, the use of this technique in clinical microbiology laboratories is growing in order to replace or complement conventional laboratory identification techniques as it provides easy, rapid, high throughput, low-cost, and efficient identification techniques. The mass spectrometer is a powerful analytical tool for both quantitative and qualitative applications. They are increasingly being used in the field of pharmaceuticals as it offers new instrumentation and ionization techniques that can be used to solve various difficult bioanalytical problems, thereby driving the growth of the mass spectrometer market globally. Mass spectrometer is used for the optimization and monitoring of fundamental chemical processes used in the manufacture of various pharmaceutical and life science products. Key trends prevalent in the mass spectrometer market are the use of refurbished instruments, miniaturization of mass spectrometers, increasing investments by governments and research institutes, the launch of technologically advance mass spectrometers, and increasing research activities.
MALDI-TOF is expected to continue to be the leading segment globally attributing to the growing use of MALDI-TOF spectrometers for various applications in clinical diagnostics, environmental and taxonomical research, and food processing and quality control. MALDI-TOF segment is projected to expand at a considerable CAGR of 8 percent. The application of mass spectrometry in biological science has progressed over the last decade. With the help of FIB system cells, biomaterials and their interfaces can be analyzed, imaged, or prepared for various techniques such as fault-tolerant operation, ensuring high reliability for closed-loop control in harsh industrial environments. The liquid chromatography-MS segment is projected to expand at the highest CAGR owing to the growing adoption of high-performance liquid chromatography in order to separate, identify, and quantify each component in a mixture during the production process of pharmaceutical and biological products.
North America accounted for the highest revenue share in the past due to the concentration of leading manufacturers, high usage of mass spectrometry in the pharmaceutical sector, and growing funding to favor the mass spectrometry market. In North America, market players are continuously taking efforts to introduce advanced mass spectrometer products. Furthermore, growing laboratory automation is helping the North American market to generate significant revenue which is expected to expand at a significant CAGR of 8.3 percent. In a number of European countries, high penetration of mass spectrometers in industrial and non-industrial research sectors and technological advancements are fueling the growth of the mass spectrometer market. The market in Europe is likely to witness significant CAGR growth. The UK contributed the highest share to the Europe mass spectrometer market. Growing food safety concerns and increased investments for pharmaceuticals has escalated the growth of the mass spectrometer market in the UK.
The mass spectrometer market in Asia-Pacific is expected to expand at the highest CAGR of 8.5 percent. Factors such as growing technological innovations and the popularity of conventional biological laboratories and increasing R&D investment in the pharmaceutical industry are expected to drive the Asia-Pacific market. Demand for mass spectrometers in APAC is likely to remain concentrated in China and Japan due to the high petrol and natural gas production and growing biopharmaceuticals and biotechnology research and development. The market in Japan is estimated to expand at a significant CAGR due to the growth in the pharmaceutical industry and growing adoption of advanced technologies. In Middle East & Africa (MEA), market players are increasing their investments to expand their physical presence. GCC countries are expected to expand at a significant CAGR due to the development of the biotechnology industry in the region. In South America, Brazil is expected to hold a significant portion of revenue for the market.
While the opportunities in the global market are expanding fruitfully, the shares are moderately consolidated among a small pool of players. Market leaders, such as Thermo Fisher Scientific, Shimadzu Corporation, and Agilent Technologies, have a strong product portfolio and are frequently indulging in mergers and acquisitions to stay ahead of the curve. Although the entry-barriers are quite high for new entrants, some of the emerging players are eating into the global shares of the mass spectrometers market via their niche technologies. Some of the other key players in the global market include Bio-Rad Laboratories, Waters Corporation, Bruker Corporation, PerkinElmer, Charles River Laboratories International, bioMérieux, and AB Sciex (Danaher Corporation). Players are introducing technologically advanced mass spectrometers and establishing partnerships with other players to meet the continuously growing demand for accurate and real-time measurements.
Technological advances
Advancements in MS technologies have enabled an ever-broadening view of the research landscape – from small molecule metabolomics to large-scale proteomics and beyond. New technologies may serve a significant role in shortening the path from biomarker discovery to validation. The technological advancements will also enable faster, more complete protein sequencing, identification, and quantitation for applications such as biopharmaceutical development and diagnostics. The years to come will undoubtedly bring new discoveries and further validation of these technologies. Innovative MS solutions will continue to provide research with an ever-greater vantage point towards molecular medicine and beyond.
MALDI-TOF MS. This technology reduces the time needed to definitively identify an unknown bacterial isolate to the species level form about 24 to 36 hours in routine laboratories to about 5 minutes, with accuracy approaching 16S rRNA gene sequencing and additional savings in manpower and reagent costs. Practically, when compared to other traditional techniques, microbial identification by MALDI-TOF MS offers a number of major advantages. In terms of efficiency, the current commercially available platforms are easy to use and require significantly reduced technical manpower in the clinical laboratory compared to sequential staining and biochemical phenotyping. This also potentially translates to improved turnaround time, earlier identification results, and better clinical care. In the near future, with improvements in species- or strain-specific marker identification, spectral signal processing technology and enhanced sensitivity of MALDI-TOF MS instruments, this versatile phenotypic identification tool can potentially be more widely applied in microbial identification in mixed or contaminated culture, sensitive detection from culture broth, and even direct identification of pathogens from blood and other body fluids without the need of bacterial or fungal culture.
LC-MS. Commonly used as LC-TOF-MS and LC-MS/MS, LC-MS has seen much growth in its clinical use in the last two decades. The separation power of LC is combined with the sensitivity and specificity of MS; the result is the simplification of sample preparation and an increase in the spectrum of detection of analytes, compared with predecessor technologies. The use of TOF-MS provides two layers of specificity on top of chromatographic separation; first, high-resolution MS combined with isotopic distribution allows determination of elemental composition (i.e. the molecular formula) of the compound of interest; and secondly, the use of in-source fragmentation by operating the ESI at high aperture voltage allows tandem-in-time mass spectrometry and detection of product ions with high mass accuracy. LC-MS have been applied in applications such as urine toxicology screening and endocrine testing of glucocorticoids, mineralocorticoids, biogenic amines and metanephrines and sex steroids. These applications often reflect the combined advantage LC-MS over alternative technologies such as higher specificity, higher sensitivity, and eliminate the need for an extra derivatization step to enhance analyte volatility and thermo-stability.
GC-MS. It has long been used in the clinical laboratory; its use for clinical diagnosis can be traced to the 1970s, with the early focus being on poisoning and inborn errors of metabolism. While it is no longer possible to say that GC-MS is the most widely used application of mass spectrometry in the clinical laboratory, the superior separation and non-selective nature of detection explains the continuing use of GC-MS. Coupling mass spectrometers with GC systems allows separation and subsequent determination of components of highly complex mixtures with a high degree of certainty. Recently, manufacturers of mass spectrometers, particularly spectrometers coupled with GC systems, have significantly reduced their overall size and have increased durability. These changes allow what was once a laboratory bench top instrument to be portable (or transportable), and sufficiently rugged to perform field analysis.
FT-MS and MRMS. Fourier-transform mass spectrometry (FT-MS) has been a sentinel technology for ultrahigh resolution intact protein identification in complex samples. Last year saw the introduction of a new technology meant to remove bottlenecks of existing platforms and help drive even higher resolution and accuracy for top-down proteomics. Now commercially available, magnetic resonance mass spectrometry (MRMS) is designed to deliver a new benchmark in mass-resolution (>20,000,000), without the need for liquid cryogens and the excess space requirements of FT-MS devices. Performance-wise, the MRMS platform allows isotopic fine structure (IFS) analysis to easily determine exact elemental compounds in complex biofluids. In a biomarker context, advanced native protein identification and label-free quantitation can be performed. Ultrahigh resolution (>20 million) and mass accuracy (600 ppb) equate to high-fidelity biomarker quantitation direct from biological samples. The ability to swap sources (ESI, MALDI, and ETD) provides greater flexibility and broader sample coverage. The novel flow injection analysis (FIA) enables large cohort, high-throughput analyses.
Automation. Automation, as applied to MS-based assays, involves automation in the management of specimens, sample preparation, the analytical run, data processing, and automated uploading of data to a laboratory information management system (LIMS). In the past decade, there have been dramatic advances in the automation of clinical MS through automated sample preparation and the ability to interface mass spectrometers with LIMS. In the clinical laboratory, the choice is often between using general purpose liquid handling systems and purpose-built sample preparation units, either standalone units or integrated on-line into the chromatography/mass spectrometry suite. Connectivity of mass spectrometer suites to the LIMS in the clinical laboratory enables processed data to enter the hospital database accessible from the clinical management system without transcription by the operator which saves operator time as well as reduce transcription error and enables central review of quality control data with other analyzers in the laboratory.
Networking and the cloud. With massive amounts of data in hand, sifting through it is no trivial task. MS labs need software with significant processing power and very fast data access to work with large data files. While computing networks that connect files and processes are more widespread in other areas, working with such large data sets across a network has, until recently, not been a viable option for MS studies. A networked chromatography data system (CDS) provides the possibility to control MS instruments and query, compare, and trend large volumes of MS data held in an optionally cloud-based central repository. Including a CDS and a LIMS in a fully integrated data-management solution can securely capture, store, and archive MS data for present and future use. Enhanced systems are able to take advantage of cloud services, enabling access to more data as well as delivering improved sharing and archiving capabilities. The new breed of software platforms bring the benefits of networking and the cloud to MS-based research and can expand the usability of large data files, allowing movement and sharing of data for further analysis or interpretation. More importantly, MS labs can make use of additional software on the network for application-centric analysis.
Outlook
In the past decades, the healthcare industry has seen revolutionary changes in the clinical laboratory, from advanced automation and laboratory informatics systems, widespread use of molecular techniques and most recently, MS-based methods. From the vantage point of time, this sequence of events seems, at least retrospectively, logical and well-anticipated. The invention of immunoassay fueled the surge of automatic analyzers. Breakthroughs in engineering and computer science accumulated in the genetic era eventually led to the genomic age. While it is beyond doubt that the importance of MS-based techniques in clinical laboratories will continue to grow, one cannot predict its growth trajectory with certainty. Will MS-based techniques merge with the next-generation DNA sequencing techniques to give rise to efficient and economical sequencing solutions applicable to the service laboratory? Will MS-based techniques attain an adequate level of automation and ease-of-operation to replace the majority of the chemical and immunological analyzers in the clinical laboratory? Will MS-based techniques see breakthroughs in miniaturization which allow them to move, literally, from bench to bedside, to serve as point-of-care testing devices?
One should stay vigilant, not only because MS-based technologies have the potential to replace current technologies and existing expertise and instrument may undergo rapid evolution, but also because the emergence of this technique is linked intricately to the generation of massive data – similar to the early days of next-generation DNA sequencing. Change-embracing yet analytical minds are required to unleash the full potential of this rapid-dominating platform and at the same time establish quality assurance measures to ensure accuracy of laboratory results and patient safety. Need for improvements in sensitivity, precision, and accuracy of instruments will largely drive the remarkable advances in instrument design. In the future, the market is expected to see greater use of miniaturized systems that can be implemented directly into automated testing processes. Direct ionization techniques are also likely to mature from the early research-focused products to more mainstream solutions geared toward non-expert users.
With MS becoming more widely used, the requirement for user-friendly technologies that meet the needs of non-MS specialists does not simply relate to instruments themselves. The analysis workflows and software used to control them is also set to become increasingly accessible and here, computer algorithms are likely to take up the slack. The volume and complexity of data being generated by high-resolution mass spectrometers will create a push for new software tools and new databases that will comprehensively support and simplify data processing and interpretation across multiple instruments and applications. The biggest change that is expected over the next three to five years is the incorporation of artificial intelligence into MS data analysis, enabling the searching of not only well-organized databases but also external data such as journal text. This will greatly increase the knowledge generation from MS data sources. Moreover, the new mass spectrometry tools will also help to minimize or even eliminate the complex sample preparation steps that are a current bottleneck in many MS workflows. The drive to increase sensitivity while pushing detection limits down will continue to be a major goal for instrument manufacturers. For now, the focus continues to be providing labs with simplified, yet powerful mass spectrometers to help them answer research questions today so they can solve complex problems for tomorrow.
Industry Speak
The Next Big Thing – MS Applications In Routine Diagnostic Laboratories
Yoshiyuki Fujino
Managing Director
Shimadzu Analytical (India) Pvt. Ltd.
The mass spectrometry (MS) is a tool with great specificity and sensitivity. With the use of chromatography as sample introduction system to MS, it gets much better separation and isolation capability. The gas chromatography (GC) with MS or liquid chromatography (LC) with MS have been successfully employed as the best analytical tools in many different fields including clinical diagnostics. ICP-MS has been used for heavy metal analysis such as Lead (Pb) in clinical laboratories.
The advancement of MS technology along with the development of new applications will only accelerate the incorporation of MS into more areas of IVD. In a clinical laboratory, the various types of MS have impacts which includes conformation of immunoassay-positive drug screen, identification of inborn errors of metabolism, analysis of steroid hormones, microbial identifications, and identification of toxic metals.
All above applications are very critical in diagnosis. However, IEM/NBS are more important in the present contest. IEM are disorders caused by gene defect which might be visible after the birth or within a few days or weeks after the birth. Tandem LCMS is used for NBS and GC-MS is used for confirmation of some of the disorders. In US, Europe, and in the developed countries, new born screening is mandatory. In India it is not. The cost of test, expertise, and the facilities are not yet available adequately for the large population in India to be tested and diagnosed. However, for creating India to be strong, it is essential to have NBS mandatory.
Varied types of clinical samples analysis using MS have increased its implication in IVD. The goal of these techniques (LCMS/MS, GCMS/MS, and ICP-MS) is to capture information of various biomolecules using targeted approach. New horizons and trends indicate a bright future of MS in IVD, MS based methods are an essential component of diagnostics laboratories and will continue to grow in near future. With the increasing focus on MS the next big thing will be MS applications in routine diagnostic laboratories.
In current future, more fast, accurate methodologies using MS will be introduced for diagnosis and for prognosis. This will make healthcare industry more robust and diverse in near future.
Second Opinion
Applications Of Advances In Mass Spectrometry In Biology And Medicine
Dr Shaik Mohammad Naushad
Head, Biochemical Genetics and Pharmacogenomics
Sandor Specialty Diagnostics Pvt Ltd
The advances in electrospray ionization (ESI) and matrix-assisted laser desorption ionization (MALDI) expanded the horizons of a newborn screening program for the diagnosis of a wide spectrum of inborn errors of metabolism covering aminoacidopathies, organic acidurias, fatty acid oxidation defects, and lysosomal storage disorders. Hydrophilic interaction chromatography-mass spectrometry (HILIC) facilitated simultaneous quantitative measurement of amino acids, neurotransmitters, purines, and pyrimidines, and also emerged as a powerful tool in the discovery of disease biomarkers. Supercritical fluid chromatography-mass spectrometry (SFC-MS) using carbon dioxide as the supercritical fluid revolutionized analytical chemistry by simultaneously separating polar and non-polar compounds within a short span. This was made possible due to lower viscosity and high diffusivity of the supercritical fluid thus facilitating higher flow rates and lower pressure drops.
In diseases such as cancer, mere detection of abnormal metabolites may not be informative and there is a need to assess localization of these metabolites in order to understand tumor microenvironment. In such situations, mass spectrometry imaging (MSI) provides insights on the in situ distribution of metabolites or biomarkers directly in tissue sections or cells. Four different ionization methods are available for such applications. MALDI MSI provides better image resolution for such applications if the selected matrix can form good co-crystals with tissue biomolecules without affecting their localization. Ambient pressure ionization of desorption electrospray ionization (DESI) MSI facilitates matrix-free ionization wherein multicharged ions can be easily detected. Laser ablation electrospray ionization (LAESI), which is a mixed ambient ionization source grounded on mid-infrared laser ablation with charged droplets produced by ESI, is effective in imaging biological molecules with high water content. Direct analysis in real time (DART)-MS enables the rapid analysis of solids, liquids, and gases at atmospheric pressure without the need for sample preparation. DART produces electronically or vibronically excited-state species from gases such as helium, argon, or nitrogen that ionize atmospheric molecules. In view of these varied applications of mass spectrometry, these technologies have become an integral part of biology and medicine thus facilitating a better understanding of biological processes and pathophysiological mechanisms.












