MB Stories
Private hospitals scramble for ventilators
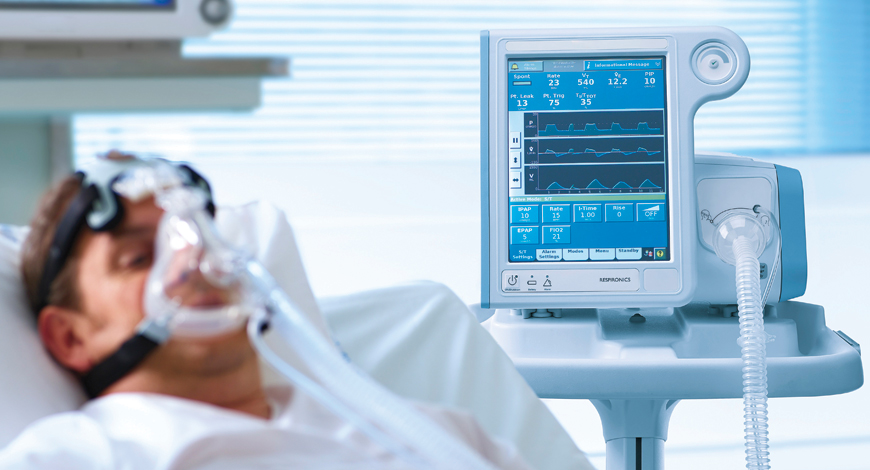
In contrast to the first wave, the Center seems well equipped for the second one. It is the private hospitals and the states seeking ventilators now.
With the second wave of COVID-19 raging and testing the Indian healthcare infrastructure, ventilators are once again being desperately sought. As the hospitals buckle under a record surge, the manufacturers are scrambling to meet demand, requirement being for immediate supplies and installation.
But there is a major difference. The central government, a major buyer in the first COVID-19 wave is not inviting bids. By December 2020, the health ministry had delivered 36,433 ventilators to government hospitals in the country, and the Directorate is done with the procurement of this product for the moment. The total installations pre-COVID were 16,000 ventilators.
Inquiries are being raised by the state governments and private hospitals for procuring the equipment. The tenders being invited by some states have stringent conditions as delivery within 7 days and penalty clauses in case of default, that is keeping some vendors away from participating. Hospitals at Maharashtra, Punjab, and Gujarat are the ones with maximum demand. In April, Maharashtra has seen an increase in demand of about 50 percent, Gujarat 35 percent, and Punjab 15 percent over last year. As Delhi witnessed another surge in coronavirus infections, according to the Delhi Corona app, on April 28, out of 1618 COVID-19 beds with ventilators, 1616 were occupied, and only two were available.
Horror stories are aplenty. Surat Municipal Corporation authorities have ordered a probe, after more than 20 ventilators from Valsad Civil Hospital were loaded in a vehicle used to transport trash and was taken to SMIMER Hospital. A video showing ventilators, that were not packed properly, being transported in such vehicles, went viral on social media. In Punjab, ventilators are gathering dust in the state government’s warehouse. The central government had sent 290 ventilators for the state in 2020, but the health department is yet to unpack them for use. Ideally, these ventilators should have been sent to medical colleges or other centers offering L-3 care for COVID patients.
Initiative is coming from many quarters. Corporate chains have taken this as a part of their CSR activity and are supplying 300-400 units of the machines to various hospitals. For instance, Flipkart and Give India Team made 28 ventilators available to the health department of the Maharashtra government.
COVID centers are also being planned. One such joint effort is being planned by Medanta and a couple of other hospitals in Pune, that are inquiring for turbine-based ventilators.
The vendors are scrambling to supply as many as they can manage. Customers want it, in running condition, like yesterday.
Skanray has made a delivery commitment of its entire production for the next 5 months or so. Buyers are paying in advance to ensure that their deliveries arrive in time. Most of the orders are coming from private hospitals in Maharashtra and Odisha.
Other brands too are very busy. On an average, in April, 500 units each are being supplied by the larger brands, such as Getinge, GE, Vyaire, and Hamilton. The other brands like Allied Medical, Aeon Medical, Mindray, Resvent, Schiller, BPL, Meditek, Philips, Nidek, Air Liquide, and Covidien are also doing their best, as many supplies as they can manage, from their parent companies.
Once the first COVID wave started to wane, the manufacturers’ inventory had started piling up. And, as the COVID cases, once again picked up end March, the supplies were made without delay, and the hospitals did not face any shortage.
Down memory lane. Demand estimated in 2019, pre-COVID was a mere 8500 units. In May 2020, the Technical Committee constituted by the Empowered Group-3, created under convenorship of Dr PD Waghela, Secretary, Pharmaceuticals, Ministry of Health and Family Welfare projected that India would need at least 75,000 ventilators till June 30, against 19,398 units available in the country, then.
Orders were placed for 59,884 ventilators on domestic manufacturers and 1000 units on overseas manufacturers. The major domestic players included Skanray (in collaboration with Bharat Electronics Limited) for 30,000 ventilators, AgVa (in collaboration with Maruti Suzuki Limited) for 10,000 ventilators, AP Medtech Zone for 9650 ventilators, and Allied Medical 350 machines.
Ventilator capacity went up from about 300 units to 30,000 units per month and from eight manufacturers to 16 manufacturers. For instance, Trivitron Healthcare expanded its manufacturing capacity to 60,000 units, and Skanray to 120,000 units per annum.
In the first wave, Skanray had supplied 30,000 units in collaboration with BEL. This was a one-time technology transfer agreement. The union health ministry had been given a budget of ₹2000 crore for 50,000 Make in India ventilators from the PM Cares initiative. The duo had manufactured the entire order in about 8 weeks, and now if required, can hike up the capacity to a much higher level, and deliver within 4 weeks or so.
Skanray also supplied 4000 units of its indigenously manufactured, non-invasive ventilator, Respiro Plus. The price had been frozen at ₹560,000 per unit, including GST and a 2-year warranty.
Manufacturers from companies other than the medical industry had been invited to meet the severe shortage of ventilators. Maruti, Mahindra & Mahindra (M&M), and Tata were the prominent ones. However, the experiment seems to have not been so successful. For instance, M&M had a transfer of technology agreement with Skanray, but not having received the certification, had to stall the project. A similar fate seems to have befallen the PSUs, automobile companies, research organizations, and start-ups that had stepped up to meet the impending challenge.
The Defense Research and Development Organization (DRDO) had requested ITI that it would transfer necessary technology and the PSU produce portable ventilators in its Bengaluru facility. CMD, ITI contended that while the company has the capabilities to produce the ventilators quickly, it would need to come up with the product prototype, after which it would take 30 to 60 days. Component sourcing would present a challenge. “ITI will need to source components locally and globally, which will likely be a cumbersome task as a result of the current lockdown,” said RM Agarwal, CMD, ITI Limited.
Worldwide too, some experiences have been similar, while others very interesting. Take UK, for example. Numerous firms from the aerospace and defense sectors, and even Formula One, offered their services. Domestic appliance maker Dyson, defense contractor Babcock, and the Ventilator Challenge UK consortium (including leading firms such as Airbus and Ford) have all received orders to make thousands of new ventilators to meet the government’s target of an extra 30,000. Rather than simply helping scale up production of existing products, these firms are working with designs that have never before been used or tested in real settings.
The first batch of devices from the Ventilator Challenge UK consortium are just 30 units. Apparently, not just anyone can make a medical device. Also, manufacturers have to be registered with the relevant regulator. In the UK, that is the Medicines and Healthcare products Regulatory Agency (MHRA). Similar regimes apply in the EU, USA, and other established healthcare markets.
Across Europe, ventilators fall into class IIb, the second highest regulatory classification. As such, manufacturing them is not as simple as just making a machine that works in a laboratory. The ongoing production of these devices has to adhere to strict rules. In this time of crisis, some rules have been relaxed. But any new ventilators still have to meet strict specifications and pass hard test protocols.
Medical device companies usually take a long time to get going. They need to build their manufacturing knowledge and supply chains to ensure their products are safe and packaged in a sterile way. They need to understand things like biocompatibility and materials made from animal by products, in order to minimize risk of transmissible diseases, such as CJD. These companies also need to develop the necessary skills in specific risk management and quality procedures.
So, starting from scratch is not an easy thing to do. The simplest approach to increase ventilator numbers is for a government to contact existing ventilator manufacturers and understand what is needed to increase production rates. For example, there may be issues with the supply of materials, a need for new machine tools, or a lack of funding needed to order thousands more components.
These companies already have the respective approvals and quality procedures in place. Efforts should be made to determine the bottlenecks that inhibit increased production, and then find the solutions. An existing provider, with a working device and functioning procedures, does not need to support a learning curve and is the best first point of call.
Another example is that of the University College London Hospital – Mercedes group, which reverse engineered an existing product design and moved to rapid production. The same sort of post-production trials apply. The simple reason for such trials is that any significant change to the software, design, or production of an existing device requires a new regulatory sign off, for which trials may be demanded. Even in times of crisis anyone’s life cannot be risked just for the sake of speed – someone will be liable in the event of death or injury.
So why modify an existing device to meet production demands instead of ramping up current production? In some cases, the two are linked: some of the manufacturers in the Ventilator Challenge are using additive manufacturing (often referred to as 3D printing) as a solution. This, in itself, is a major design change and has to be formally signed off, not least because additive manufacturing is a highly complicated set of techniques – one cannot just put any design into a 3D printer and press go.
New techniques, then, are bringing different skills and companies into play which are being married with traditional medical devices knowhow.
If, however, the manufacturer or any of its supply chain is not within the country there is little a government can do to help. In the UK’s case, successive governments since the 1970s have overseen a relative decline in manufacturing, including in the medical devices, sector, its associated supply chain and skills base. As a consequence, the UK is more reliant on overseas subcontractors.
This pandemic has highlighted the failings of this strategy. If a country does not have the capabilities to build its own equipment, then it is exposed in a global crisis. Perhaps this outbreak will wake up countries to the notion that certain things have to be essential items in a country’s economic portfolio. The coronavirus has reminded us that food, medicines, and medical devices and their respective supply chains are most certainly essential items.
NASA engineers have developed a new, easy-to-build high-pressure ventilator tailored specifically to treat COVID-19 patients. VITAL (Ventilator Intervention Technology Accessible Locally), passed a critical test mid-April at the Icahn School of Medicine in New York, an epicenter of COVID-19 in the US.
VITAL is designed to treat patients with milder symptoms, thereby keeping country’s limited supply of traditional ventilators available for patients with more severe COVID-19 symptoms. “We specialize in spacecraft, not medical-device manufacturing,” said Michael Watkins, Director of NASA’s Jet Propulsion Laboratory. “But excellent engineering, rigorous testing, and rapid prototyping are some of our specialties. When people at JPL realized they might have what it takes to support the medical community and the broader community, they felt it was their duty to share their ingenuity, expertise, and drive,” Watkins said. NASA is now seeking FDA approval for the device via an emergency use authorization.
The new device would not replace current hospital ventilators, which can last years. Instead, VITAL is intended to last 3–4 months and is specifically tailored for COVID-19 patients. “Intensive care units are seeing COVID-19 patients who require highly dynamic ventilators,” said JD Polk, NASA’s chief health and medical officer. “The intention with VITAL is to decrease the likelihood patients will get to that advanced stage of the disease and require more advanced ventilator assistance,” Polk said.
The hurried supplies made by some brands in March and April 2020 have issues as some models did not incorporate all the requisite features. Swami Ramanand Teerth Rural Medical College, Ambajogai complained that from the 27 ventilators that it procured, only one disposable flow sensor had been supplied with every machine. Another common complaint was of the machines not getting power supply. Many machines from that lot of delivery are now stacked away at the hospitals and not being used.
 Ashok Patel
Ashok Patel
Founder & CEO,
Max Ventilator
“Now that ventilators are included in the MDR 2020 list, effective April 2020, within 18 months all ventilators will need to follow the requirements of ISO 13485; and comply with applicable BIS/ISO product standards within 42 months.”
A major issue is lack of trained manpower to operate the machines. The country has 10,000 intensivists as against over 50,000 ventilators installations. Trained ICU specialists and respiratory technicians are needed to operate the machines. Many centers are resorting to treating patients, using the ventilators in a non-invasive mode. In one case, Karnataka state government had let out ventilators to private medical establishments for 6 months for government-referred patients, at a charge of ₹500 per day, the private hospitals’ challenge was the manpower to handle the additional equipment.
The Indian government is attempting to give a push to domestic manufacturing and attract large investment in medical device sector. The Department of Pharmaceuticals is part of the Production Linked Incentive (PLI) scheme launched for promotion of domestic manufacturing of medical devices to ensure a level playing field for the domestic manufacturers of medical devices. A total financial outlay of ₹3420 crore for the period 2020-21 to 2027-28 has been allocated for medical devices. And ventilators are included in this scheme. So far, for this product segment, only GE has come forth with a committed investment of ₹53.86 crore, the amount includes patient monitors too.
The commercial production is projected to commence from April 1, 2022 and the disbursal of the incentive by the government over the 5 years period would be up to a maximum of ₹121 crore per applicant per target segment. A total of ₹729.63 crore has been made for the four target segments of medical devices identified so far. Some applications are pending and proposed to be taken up for approval by end of February 2021. The setting of these plants will make the country self-reliant to a large extent in the specified target segments in the medical devices sector.












