MB Stories
Recovery in 2021 – The light at the end of the tunnel

2021 saw the sector recovering from the pandemic doldrums. This trend is expected to continue, though there could be one potential brake on growth on the regulatory side.
The year of 2020 was beyond description, surreal, and overwhelming, to say the least. The health crisis greatly challenged health systems, profoundly affecting the world. Healthcare equipment industries, which were under the spotlight during the crisis, were in a more complex situation. The volatility of the pandemic made it difficult to gauge how long it would last and how it would affect the diverse healthcare equipment industries. Now, just over 20 months after the World Health Organization declared COVID-19 a pandemic and the nations worldwide declared it a national emergency, the data show that MedTech has weathered the global operational disruption and entered a period of recovery and renewal.
Over the course of 2020, the industry’s revenues grew for the fourth consecutive year (+6.3%), with the non-imaging-diagnostics segment recording a particularly impressive 24-percent annual growth rate, largely from the pandemic-related demand. The global MedTech market is estimated at USD 519.39 billion in 2021 and is expected to reach USD 531.77 billion by 2023. This growth is mainly attributed to medical equipment sectors that were exempted from the pandemic’s effects or even flourished because of the pandemic.
CT imaging procedures were widely adopted to diagnose, monitor, and triage patients with pneumonia as a result of COVID-19 infection. In 2020-21, governments provided COVID-19 relief to healthcare systems through funding and large equipment tenders. Funding for CT systems was often included in these stimulus packages. The sales of CT imaging equipment were boosted.
Molecular diagnostics can detect a large battery of viral and bacterial pathogens rapidly in a single sample, thus significantly improving the yield of detection. The molecular diagnostics market soared during the pandemic owing to high demand for PCR testing.
Antibody (or serology) tests can help tell if a person has immunity to COVID-19. Hematology devices used for serology tests soared during the pandemic, offsetting declines in other hematology instruments, caused by drastic reductions in both clinic and research hematology diagnoses.
Relevant medical equipment that combats the pandemic had significant revenue growth (76%) in 2020, with ventilators (300%) and molecular diagnostics (200%) having particularly high growth.
The global demand for electric beds significantly increased during the pandemic. The pandemic demanded a dramatic change in hospital infrastructure to manage the critical care and rehabilitation needs of COVID-19 patients. Over the course of the pandemic, it was found that prone positioning helped increase the oxygen intake of COVID-19 patients, so the use of ventilators could, at times, be avoided.
However, without the necessary equipment, five people are needed to turn a patient to prone positioning, which causes continuous disruptions and strain for healthcare personnel. Electric beds both free up medical resources and provide opportunities for better care.
Some sectors were resilient during the pandemic.
Most consumer medical devices industries had surprising growth in 2020 owing to reduced hospital visits and increased home care demand. Patients use CGMs and insulin pumps remotely at home, so the pandemic did not affect the market as much as others. Instead, the market kept growing, driven by aging demographics and an increasing prevalence of diabetes diseases.
Unlike most consumer medical devices, COVID-19 hit the hearing aid and electric wheelchair and scooter industries hard. Face-to-face consultations and fitting activities to sell hearing aids and the need for mobility through wheelchairs and scooters fell substantially because of restrictions imposed during the pandemic to avoid virus spread via human contact.
While some MedTechs, particularly those reliant on elective procedures that were deferred as the crisis broke, took a hit during the pandemic, even these companies witnessed resurgence from the second-half of 2020 onwards as procedures resumed. Notwithstanding further disruption from the Delta variant, companies reliant on elective procedures, seem set for accelerated growth as surgeries and other elective procedures get back on track. Indeed, 94 percent of the commercial leaders and conglomerates that reported their first-half financials for 2021 have improved their revenues compared to 2020.
MedTech’s strong fundamentals are reflected in investor sentiment for the industry – market capitalization has strongly rebounded since the global dip of March 2020 (outpacing big pharma and the broader indices), driven by the very strong performance of MedTech’s emerging leaders. MedTech’s emerging leaders (companies with annual revenues below USD 500 million) saw a 128-percent rise in public valuations between January 2020 and August 2021.
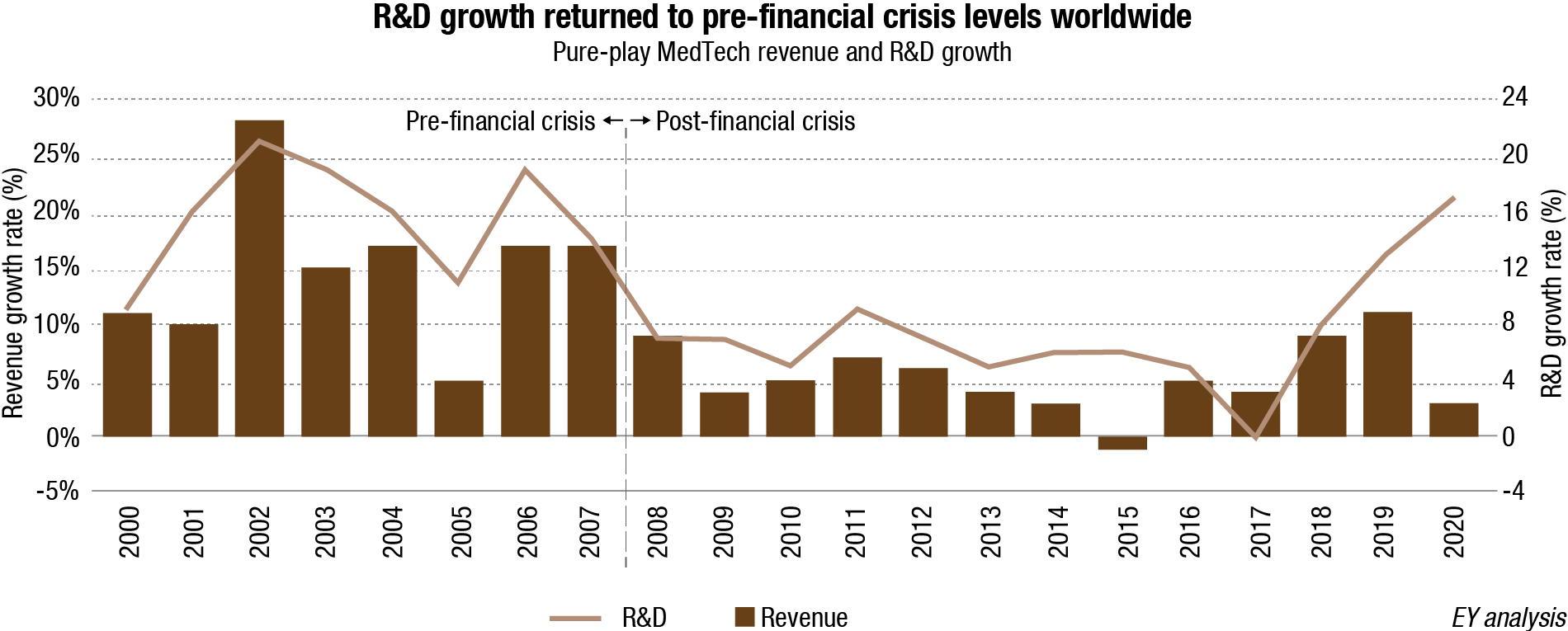
Investors’ ongoing confidence in MedTech is validated by data, indicating the health of the industry’s innovation ecosystem – specifically, the industry’s level of investment in the R&D and M&A fields, and the level of venture capital (VC) it attracts. Pure-play MedTechs reinvested heavily in R&D in 2020 – recording the largest annual growth rate in R&D spending (+17.2%) since before the financial crisis of 2007 – signaling the industry’s confidence in its capacity to keep innovating.
MedTech gears up for a vintage acquisition year
Judging by the merger and acquisition activity among medical device makers so far this year, the pandemic is a thing of the past. Over the 12 months ending in June 2021, MedTech companies executed 288 M&A deals.
The main factor behind this resurgence of acquiring is probably pent-up demand owing to the deals put on hold last year. Only 118 transactions closed in 2020, less than half the 10-year average, as buyers waited out the COVID-19 storm.
The biggest deal to close this year was done by a company keen to bounce back from the COVID-19 doldrums.
Siemens Healthineers was by no means the worst-hit big MedTech during the lockdown period, but it was hit. Its USD 16.4 billion move on Varian puts it in a commanding position in diagnostic imaging and radiotherapy just as hospitals are reopening for more routine procedures, in which these technologies are used.
Other deals look in the other direction. Philips bought Biotelemetry for its remote patient-monitoring technology, reasoning that the shift to telehealth, already apparent before the pandemic, but very much accelerated by it, is here to stay.
Boston Scientific announced a USD 1.3 billion buy-out of Preventice Solutions, which designs remote-monitoring services and mobile health solutions for cardiac arrhythmia patients.
In September 2021, Baxter signed a USD 10.5 billion deal to acquire connected care specialist Hillrom.
The innovators that form the standard acquisition targets for MedTech’s larger players are dependent on continued funding to support their R&D activities, particularly in the form of VC.
Encouragingly, the industry attracted USD 9.1 billion in VC in the 12-month period to June 2021, up 34 percent over the previous year and the highest level seen in the past decade.
The crisis has not significantly slowed MedTech’s commercial progress – but could it have accelerated the industry’s evolution?
A few companies made significant gains from product lines relevant to COVID-19, from PPE to diagnostics. However, the more significant long-term impact of the pandemic may be its effect on the industry’s business models. The pandemic has shifted healthcare’s center of gravity in fundamental ways, to which the industry must now respond.
|
Diagnostic and lab equipment assets in high-demand Select US and European M&As, H2 2020 – H1 2021 |
|||||
| Acquiring company | Location | Acquired company | Location | Value (USD mn) | Buyer’s deal driver |
| Siemens Flealthineers | Germany | Varian Medical Systems | US-California | 16,400 | Build scale (oncology) |
| Steris | Ireland | Cantel Medical | US-New Jersey | 4,600 | Portfolio expansion (multiple) |
| Philips | Netherlands | BioTelemetry | US-Pennsylvania | 2,800 | Build scale (patient monitoring) |
| Roche | Switzerland | GenMark Diagnostics | US-Southern California | 1,800 | Portfolio expansion (diagnostics) |
| DiaSorin | Italy | Luminex | US-Texas | 1,800 | Portfolio expansion (diagnostics/research & other equipment) |
| Boston Scientific | US-Massachusetts | Preventice Solutions | US-Minnesota | 1,225 | Portfolio expansion (diagnostics) |
|
EY |
|||||
There are, however, reasons to believe that the rate of M&A deal-making was slow in the second half of 2021. Several publicly listed companies previously talked of as targets have seen startling growth in valuations, which might price them out of a potential buyer’s reach.
Still, deals have been relatively substantial this year, and the average deal value in the first half of 2021 was USD 1.1 billion, the largest amount for a decade.
IVD companies seek M&As to avoid COVID cliff
According to Our World in Data, on November 23, 2021, 53.4 percent of the world population has received at least one dose of a COVID-19 vaccine. Singapore currently leads with the largest share of people who are fully vaccinated against COVID-19, at 91.91 percent. This is followed by the UK at 67.69 percent, the US at 57.95 percent, Europe at 57.20 percent, and India at 29.15 percent. The expected decline in COVID-19 testing is being referred to in the IVD industry as the COVID cliff. IVD companies are preparing for a reduction in sales revenues for COVID-19 tests ranging anywhere from a steep drop-off to a more gradual downturn.
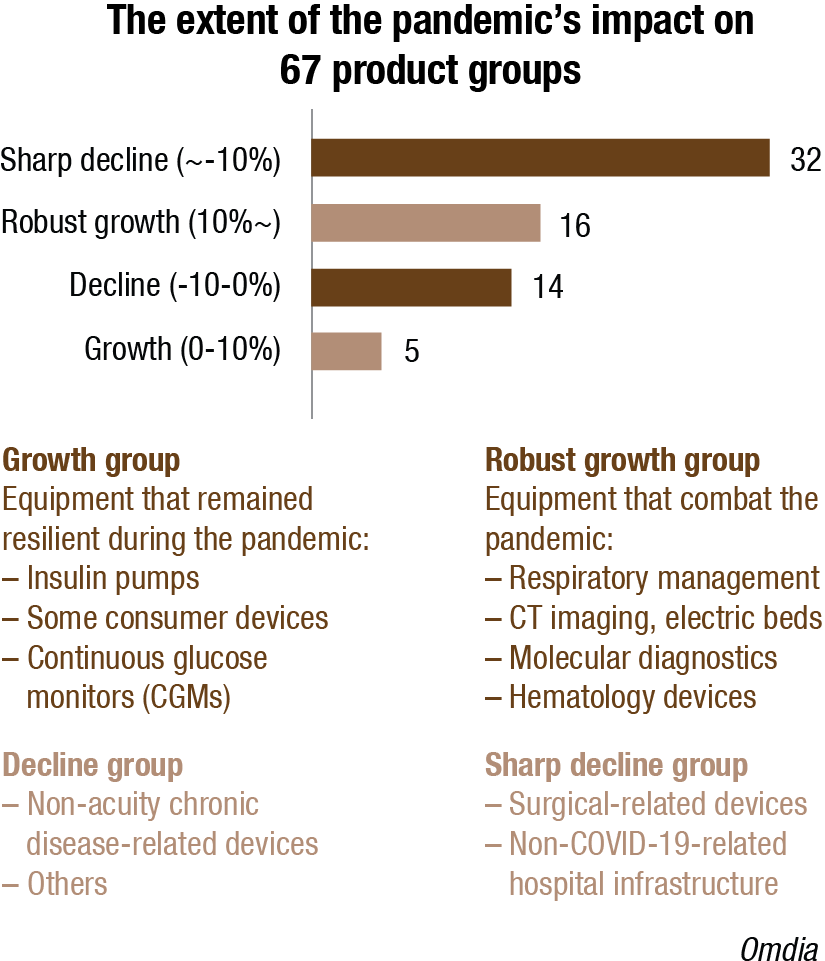
Sales related to COVID-19 tests are just one part of the picture. The Covid-19 pandemic dramatically altered the biotech landscape last year with increases in funding to biotech, most of which were COVID-related, and many IVD companies benefited from this increased funding. An unprecedented number of IVD companies entered the Covid-19 testing space, developing and launching products in record time and numbers. Clinical trial volumes also saw a significant increase last year.
In preparation for the COVID cliff, major IVD companies have turned to mergers and acquisitions to shore up their diagnostic testing portfolios.
In April 2021, Hologic made a similar move by acquiring Mobidiag, which also offers multiplex testing. Hologic said the acquisition will enable the company to enter the acute care test market, which is projected to grow after the pandemic ends. In the same month, DiaSorin agreed to buy its US rival, Luminex, to boost its core molecular business and offset the expected drop in demand for COVID-19 tests in the second half of the year.
In March 2021, Roche signed a definitive agreement to acquire GenMark Diagnostics for USD 1.8 billion to access its molecular diagnostic panel tests, which are designed to detect multiple pathogens from a single patient sample. Multiplex testing is currently gaining preference as it allows physicians to quickly diagnose a patient or rule out multiple common pathogens.
Thermo Fisher Scientific was busy with M&A in January 2021. The Waltham, MA-based company said it was acquiring Mesa Biotech, developer of a PCR-based rapid point-of-care testing platform for detecting infectious diseases including COVID-19, for USD 450 million in cash with the potential for USD 100 million in milestones.
Roche, Hologic, DiaSorin, Thermo Fisher Scientific are all strong players in the COVID-19 testing space, and these M&As indicate that the market is shifting its attention to prepare for a return to a post-pandemic world.
Floating MedTechs go big or go home
So far in 2021, the public markets have proved remarkably receptive to floating medical devices companies. Ten devices and diagnostics groups listed on Western exchanges in the first half, and the total raised, at USD 3.3 billion, is the largest six-month figure for three years.
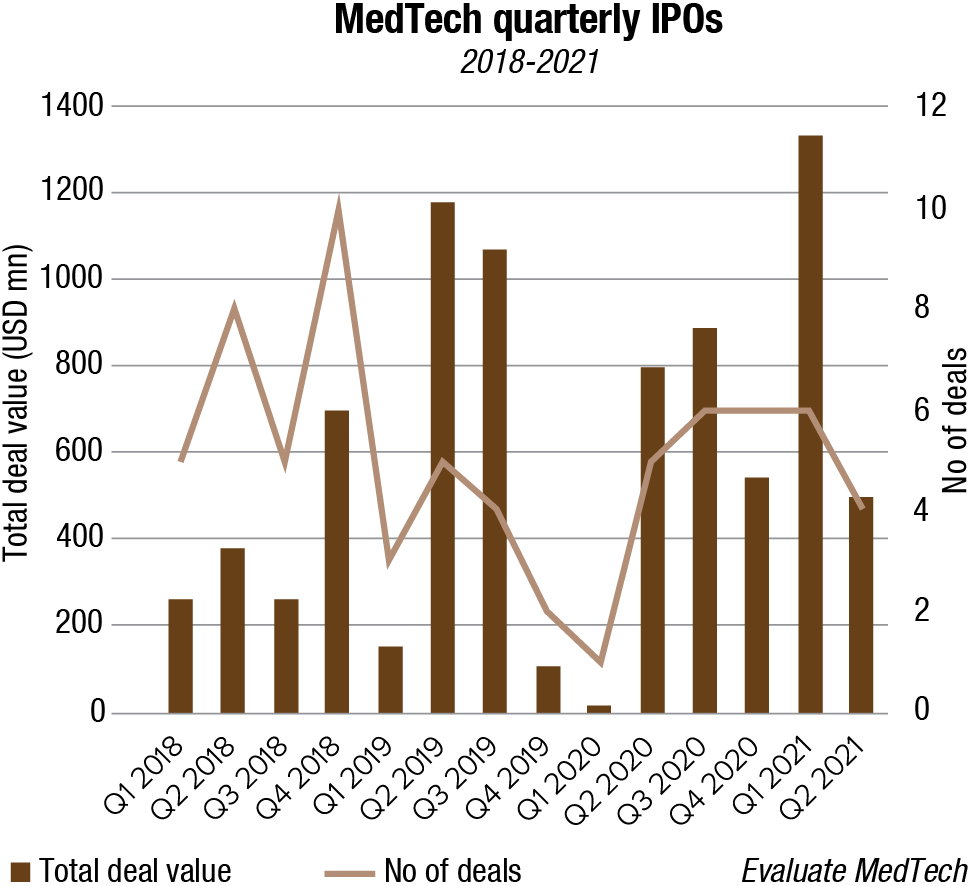
The second quarter did see a decline from the first in both the number of flotations and the amount raised, but it is surely too early to conclude that the window is creaking shut.
The first IPO of 2021 was also the biggest. Ortho-Clinical Diagnostics, formerly a J&J unit, sold to private equity in 2014, floated on Nasdaq in a deal worth nearly USD 1.5 billion. These mega-IPOs, once unheard of in the medical devices sector, are becoming more common – one billion dollar-plus IPO has occurred every year since 2016, with the exception of 2017.
Ortho-Clinical is unusual in that it had to price its offering at a steep discount to its preannounced range to get investors on board. The only other group that had to do this was the orthopedics firm Bioventus, which went out with a 13-percent haircut.
|
5 biggest MedTech IPOs H1 2021 |
|||||
| Date | Company | Focus | Amount raised | Discount/ premium | Share price change to June 30 |
| Jan 28 | Ortho-Clinical Diagnostics | IVD | USD 1.5b | -21% | 26% |
| Feb 11 | Signify Health | HIT | USD 649m | 17% | 27% |
| May 27 | Singular Genomics Systems | IVD | USD 258m | 5% | 25% |
| Feb 11 | Talis Biomedical | IVD | USD 254m | 7% | -31% |
| Feb 5 | Lucira Health | IVD | USD 176m | 6% | -61% |
|
Evaluate MedTech |
|||||
The preponderance of in-vitro diagnostics companies among the MedTechs that have gone public so far this year is a continuation of a trend that has been clear for some time; of the 19 MedTechs to float last year, six were diagnostics groups.
What is intriguing is how poorly some of this year’s diagnostics flotations have done once on the stock market. Lucira launched an upsized offering in early February at USD 17, and soon hit a high of USD 37, but shed value rapidly after that, becoming the worst-performing market debutante of the first half. It is likely that the falling demand for COVID-19 testing in the US is at least partly to blame for Lucira’s disappointing showing – something that would also help explain Talis Biomedical’s 31 USD share price fall.
The overall message for device makers wishing to float appears to be – go big or go home. All but one of the MedTech deals raised at least USD 100 million. And, even excluding Ortho-Clinical’s megadeal, the next largest MedTech IPO – Signify Health’s USD 649 million offering on the NYSE – dwarfed biotech’s biggest.
New regulatory and reimbursement models
Regulators have struggled with their additional burdens during the pandemic crisis, with the standard device-approval processes slowing. Reflecting this, 1H2021 data shows a significant drop in premarket approvals (PMAs) (12) and 510(k) approvals (1360) compared to 1H2020. A record number of PMA applications are expected by the end of this year, with the regulators viewing 2021 as a reset year and anticipating a resumption of normal approval rates in 2022.
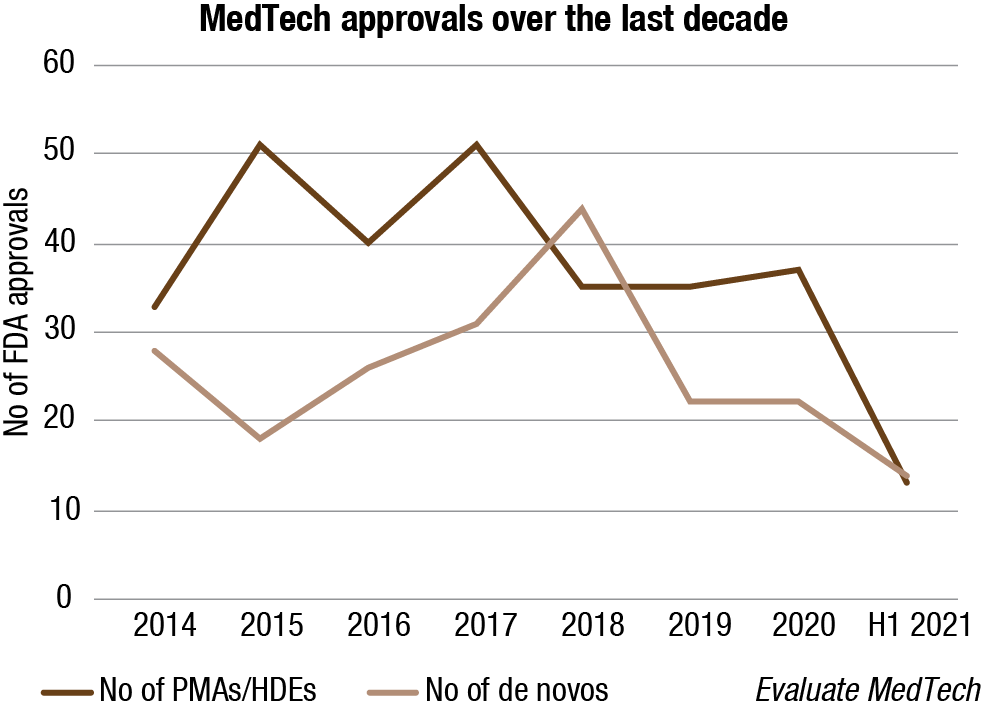
Yet, the industry now has an opportunity to seek more than just a reset in its regulatory environment. As the industry rethinks business models, it needs to work with the regulators to forge a regulatory environment that can support this evolution. During the crisis, regulators showed flexibility to accommodate the expansion of remote-care provision and allow a rapid emergency rollout of new products; the challenge for the industry now is to consolidate these changes in the legislation to underwrite the ongoing transformation of care delivery.
Other regulatory issues that will help shape MedTech’s future business model include the role of digital therapeutics, classified as software-as-a-medical-device (SaMD). Since the FDA’s April 2020 guidance relaxed the entry barriers for digital therapeutics, anticipating that they could meet the need for expanded access to therapies for mental health, approvals for digital therapeutics have followed, including:
Akili’s video game EndeavorRx, a prescription-only digital therapeutic for attention-deficit hyperactivity disorder (ADHD), was approved by the FDA via the De Novo premarket review pathway in June 2020.
Cognoa’s ASD Diagnosis Aid software for identifying autism spectrum disorders in children aged 18 months through five-year olds was approved in June 2021, also via De Novo.
Happify Health’s Ensemble therapeutic for treating major depressive disorder or generalized anxiety disorder won 510(k) approval in July 2021.
However, reimbursement pathways for this emergent class of MedTech products have yet to be clarified, for example, by CMS. In Europe, the situation has progressed more quickly since the German Digital Healthcare Act (DGV) of December 2019 came into effect.
|
Approvals by therapy area H1 2021 |
||||
| Evaluate Medtech classification | Number of PMAs/ HDEs | Avg approval time (mths) | Number of de novos | Avg approval time (mths) |
| Anaesthesia & respiratory | – | – | 1 | 10.5 |
| Cardiology | 5 | 10.4 | 9 | 13.2 |
| Ear. nose & throat | 1 | 15.0 | 1 | 12.9 |
| Gastroenterology | – | – | 1 | 7.0 |
| General & plastic surgery | – | – | 1 | 22.4 |
| In vitro diagnostics | 3 | 16.7 | 1 | 9.9 |
| Neurology | – | – | 2 | 7.3 |
| Ophthalmics | 1 | 11.1 | 1 | 10.2 |
| Orthopaedics | 1 | 7.6 | – | – |
| Physical medicine | – | – | 2 | 10.2 |
| Radiology | 2 | 10.1 | – | – |
| Total | 13 | – | 14 | – |
| Average | – | 12.2 | – | 11.5 |
|
Evaluate MedTech |
||||
One of the industry’s regulatory goals should be to make faster launches a permanent reality by embracing the FDA’s proposed total product lifecycle (TPLC) approach. Outlined in an FDA paper in February 2021, it stated, “The FDA’s traditional paradigm of medical devices regulation was not designed for adaptive artificial intelligence and machine learning technologies.” TPLC can allow AI and SaMD products into market faster, if there is a system in place for subsequent post–marketing surveillance. In the future, this accelerated model could become the norm for hardware and software devices; but making it work will depend on the industry’s ability to gather data.
Supply-chain resilience and agility
The COVID-19 crisis highlighted concerns that the supply of key devices may be vulnerable in times of global disruption. It is another area where regulatory involvement in the industry is intensifying, with lawmakers discussing the potential need for onshoring or nearshoring certain operations to bolster resilience ahead of future challenges.
At present, MedTech supply chains operate in an asset-heavy business model. They typically hold a significant amount of redundant inventory, from orthopedic spare parts to unutilized loose instruments. If the industry can better translate demand signal into supply chain reality (cutting out, for example, distortions caused by salesforce over optimism), embrace AI analytics for better forecasting, and build greater flexibility – in addition to other innovations, such as using smarter use of imaging to anticipate the size of implants required – the industry could significantly downsize its redundant inventory.
On the distribution side, there may be more scope for MedTechs to work directly with suppliers, and cut out the sales companies they now rely on as intermediaries. Upstream of these operational changes, MedTechs could incorporate integrated business planning into supply chain strategies to accommodate the industry’s long lead times for supply.
Beyond these opportunities, other supply chain challenges loom, and many MedTechs already are engaging with the need to update supply chain strategy, including:
- Baxter has deployed a cloud-based digital infrastructure to simulate demand patterns for its renal therapy devices, using data modeling to understand the location of critical patients, increase manufacturing capacity, and accelerate shopping times.
- Siemens Healthineers signed a July-2020 agreement with logistics giant DHL, focusing on digital technology, robotics, and the goal of building end-to-end, digitally-enabled supply chain solutions.
- Fresenius Medical Care signed a July-2021 deal with UPS-owned Polar Speed to help bring dialysis solutions to homes and hospitals in the UK.
With providers and regulators apparently supporting a move away from traditional clinical settings toward data–driven, home–delivered healthcare, MedTechs will need to build smarter, more agile supply chain strategies to deliver care to a patient’s front door.
2021 and the years beyond
2021 showed a sector recovering from the pandemic doldrums. The second half ought to see this continue, though there could be one potential brake on growth on the regulatory side.
In terms of M&A, the picture is fairly rosy, with more than USD 10 billion-worth of deals announced in January alone. 2021 had the strongest first quarter for device maker deal-making since 2016. Since then, the pace has slowed somewhat, but deals worth USD 11.6 billion remain open.
Many MedTechs, particularly COVID-19 diagnostics makers, are sitting on considerable cash piles and looking to spend, so it seems likely that by the end of the year 2021 will have seen a pretty healthy sum spent on acquiring innovative healthcare tech.
No retrenchment in private financings is likely, either. Private MedTechs can tap vast amounts of cash with relative ease, aided by increased participation by non-traditional venture funders, such as corporate VCs whose coffers are swollen with cash reaped during the pandemic, and players such as family offices and hedge funds.
With the public markets continuing to show enthusiasm, the buoyant IPO scene is also likely to continue for the next few months at least. In combination with the sudden explosion of special-purpose acquisition companies looking for devices makers and digital health groups to buy, the opportunities for exits are expanding, further spurring venture investors.
One uncertainty for the MedTech industry in the back half of the year will be the FDA’s possible behavior. The appointment of a new permanent commissioner could mean the pace of approvals picks up again – or it could go the other way, with more thorough scrutiny, particularly of higher-risk devices, driving approvals down.
The latter would be alarming for the MedTech sector. If companies cannot get their products to market in a reasonable timeframe, even the most generous investors and buyers will wonder whether their cash might not be better spent elsewhere.












