Daily News
Running to the lab for a venepuncture is passé
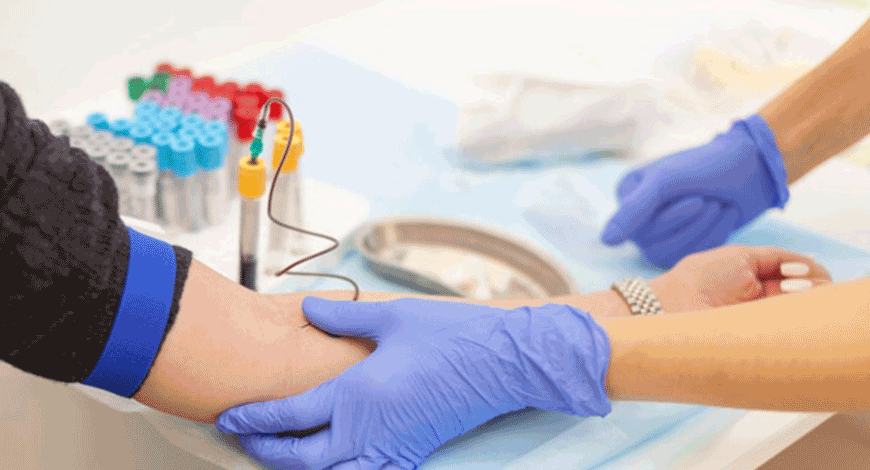
The COVID-19 outbreak is an unprecedented global public health challenge and is expected to have a significant impact on the blood collection devices market.
Both hospital and independent laboratories are reviewing each test to decide whether to recommend consultations with laboratory hematologists for tests with a higher risk profile or not offer tests that could not be performed safely. This affected the market negatively in the first few months of the pandemic, reducing the use of blood collection devices. However, increasing caution and rising testing volumes, along with the need for regular health and body checkups will ensure market growth in a later phase.
Results now take an average of four to six days, much longer than the two to three days required before. This is because tests for hospital patients and symptomatic healthcare workers are prioritized and take one day on average, which has resulted in a delayed cycle. While it has affected market growth to some extent, the situation is expected to change for the better.
Making diagnostic testing more accessible
The COVID-19 pandemic is accelerating changes already underway in the practice of medicine, spurring both innovations and growing pains around technologies that allow patients to interact with the healthcare system virtually. Larger and larger amounts of data can now be generated from smaller and smaller amounts of blood. However, problems still exist at the moment when human skin is pricked and blood is drawn or absorbed. Venipuncture remains painful, and results generated from dried blood spot (DBS) cards still suffer from certain drawbacks. It is problematic for many patients and participants, and it is costly and cumbersome for many labs. Newer micro-sampling technologies are being designed to collect quantitative, volumetrically accurate specimens.
Results from these methods correlate to those associated with wet blood, eliminating much uncertainty and concern, and creating a smarter sampling event. These technologies have the potential to catalyze innovation in precision medicine and remote monitoring, and to allow clinical labs to adopt more patient-centric approaches. A recent study was conducted to examine the possibility of micro-sampling collection devices that allow patients to self-collect their capillary samples.
The procedure requires minimal training, and can be conducted in a patient’s home or at a remote clinical site. The study’s findings indicate that most patients were at ease with self-collecting a capillary sample at home rather than going to a clinic, laboratory, or any other phlebotomy collection site.
 “For all government institutions procurement of products and services has to be done through GeM portal. This has opened opportunity to many suppliers and manufacturers to sell products but procuring a quality product at right price is a challenging job for a buyer. Non-availability of specific category for a product, clubbing of different items in a category interfere in lowest price point. Participation of suppliers with lesser credentials and from all over the country creates issues with the quality, after sales and service of the product. Several checks and balances have been introduced in the portal over the period of time but still it’s a long way till GeM becomes prefered choice for buyers.”
“For all government institutions procurement of products and services has to be done through GeM portal. This has opened opportunity to many suppliers and manufacturers to sell products but procuring a quality product at right price is a challenging job for a buyer. Non-availability of specific category for a product, clubbing of different items in a category interfere in lowest price point. Participation of suppliers with lesser credentials and from all over the country creates issues with the quality, after sales and service of the product. Several checks and balances have been introduced in the portal over the period of time but still it’s a long way till GeM becomes prefered choice for buyers.”
Dr Alok Singh, Incharge Blood Bank, Dr Hedgewar Arogya Sansthan, Govt. of NCT of Delhi
BD led the way, when it announced a strategic partnership agreement with Babson Diagnostics in February 2020. with the aim of making laboratory-quality diagnostic testing more accessible to patients. With the use of a new capillary blood collection device being developed by BD, which once approved by regulatory bodies, will circumvent the need for a trained phlebotomist to collect blood for lab testing and can be used in a retail setting. Capillary collection is a less invasive collection technique done with a lancet versus a large needle. One of the big advantages of capillary blood collection is that it can be done in settings beyond a hospital or a lab, like a retail location.
Traditional challenges with capillary blood are getting enough volume of blood, a poor patient experience, healthcare worker blood exposure, and blood quality. The system is designed to provide consistent volume and blood quality, reduces blood exposure to the healthcare worker, and most importantly provides a better patient experience. In early testing of the system, patients preferred this new capillary collection device 3-1 over traditional capillary collection techniques. In particular, because our system does not rely on the milking of the patient to get the sample, patient comfort is improved. The sample quality is superior to conventional capillary blood collection techniques and the company is targeting a product that can achieve quality comparable to a venous blood sample. In particular, preliminary testing has shown that hemolysis is significantly improved versus traditional capillary blood collection methods. The overall cost of the test should be comparable to costs today.
A device from Seattle start-up Tasso helps patients painlessly collect their blood at home. Ben Casavant and Erwin Berthier were working on microfluidics projects at the University of Wisconsin-Madison. The duo were inspired by Netflix’s DVD rental service, that was revolutionary when it launched in 1998. No longer would movie buffs have to head to their local Blockbuster to rent their desired movie. They could instead choose the title they wanted, wait a couple of days, and it would arrive in the post without them needing to leave the house. They wondered if a similar level of convenience could be applied to medicine. Casavant and Berthier imagined a future where heading to the doctor’s office for a venepuncture was as obsolete as a trip to a DVD rental store. They wanted to give patients the tools to safely and painlessly take their own blood sample whenever and wherever was convenient for them.
Tasso makes two devices: Tasso-M20 for dry blood samples and Tasso-SST for liquid blood samples. Testing kits are dispatched directly to the patient. Tasso’s devices are being validated for routine diagnostics, chronic disease monitoring, athletic testing, and clinical trials. The team needs to prove that the capillary blood collected with the device is equivalent to that collected when professionals insert a needle into a patient’s vein. Tasso has a submission with the US Food and Drug Administration and is involved in several clinical studies with organizations, such as the Fred Hutchinson Cancer Research Center and Cedars-Sinai, to expand the number of diagnostic tests for which the device can be used to collect blood. Before the coronavirus pandemic, no one could have foreseen quite how important remote healthcare services would become. Unsurprisingly, Tasso has seen increased demand for its devices in recent months. In July 2020, Tasso completed a USD 17 million Series A financing round, which will be used to scale manufacturing and operations for its devices.
TAP and Pivo recently won US FDA 510(k) clearances for blood collection devices recently. It is a push-button device from Seventh Sense called TAP, and a needle-free device from Velano Vascular called Pivo. Neoteryx, in the meanwhile is pursuing microsampling of blood but focusing on dried blood spots.
Market dynamics
The blood collection devices market, comprising blood collection tubes, blood collection needles, and blood collection sets was valued at USD 9060.98 million in 2019 and is projected to reach USD 14,228.70 million by 2027; it is expected to grow at a CAGR of 5.9 percent during 2020-2027, estimates ResearchAndMarkets.
 “There is a growing trend toward innovations in blood collection with a focus in improving patient experience and making blood draws more efficient. This is really important as blood collection is the first interface between the patient and the testing process. Manufacturers of blood collection devices have significantly improved by implementing better safety features to avoid needle stick injury, standardized needle bevel for decreased pain, plastic vacutainer blood collection tubes to ensure correct blood volume, and anticoagulant ratio, but the procedure of phlebotomy remained to be a personal skill for a long time. However, recently US firms have received FDA clearances for blood collection through robotic needle-free devices and microsampling devices.”
“There is a growing trend toward innovations in blood collection with a focus in improving patient experience and making blood draws more efficient. This is really important as blood collection is the first interface between the patient and the testing process. Manufacturers of blood collection devices have significantly improved by implementing better safety features to avoid needle stick injury, standardized needle bevel for decreased pain, plastic vacutainer blood collection tubes to ensure correct blood volume, and anticoagulant ratio, but the procedure of phlebotomy remained to be a personal skill for a long time. However, recently US firms have received FDA clearances for blood collection through robotic needle-free devices and microsampling devices.”
Dr Rajesh V Bendre, Chief Pathologist, Head Laboratory Medicine & Blood Bank Services, Jaslok Hospital & Research Centre
The blood collection equipment market is highly competitive. Becton Dickinson and Company, Cardinal Health, F.L. Medical SRL, Greiner Bio One International GmbH, Haemonetics Corporation, Henry Schein, Inc., McKesson Corporation, Medline Industries, Inc., PreQ Systems, Qiagen, continue to hold a substantial market share.
Blood collection needles. The blood collection needles/holders segment held the largest share of the market in 2019 and it is expected to continue its dominance. Nowadays, various advancements in the field of medical devices are also fueling the market growth.
For example, siliconized needles are currently being used at multiple instances, in order to minimize trauma and also to minimize risk of vein cross perforation with short bevel. Owing to the fact that a huge number of injuries occur within seconds after an invasive device is removed from the vein, presently, majority of the marketed needles provide immediate protection at the puncture site. Hence, the concerned segment is expected to retain its dominance in the global market over the next 7 years.
Blood bags. The global disposable plastic blood bags market is projected to expand at a CAGR of 9.5 percent in terms of revenue. Excess production of disposable blood bags by Indian manufacturers offers major potential to export blood bags to transitional countries in Europe and MEA. Disposable plastic blood bags have overshadowed the usage of glass bottles for collection storage, transfer, or processing of blood and blood components. This is because of convenience and mechanical strength of polyvinyl chloride (PVC) bags over glass bottles, which require exhaustive cleaning and sterilization before each use.
Increasing tender-based purchasing practices of blood collection bags by government blood banks and hospitals is expected to boost growth of the disposable plastic blood bags market in coming years. Moreover, increasing need for blood and blood-derived products is escalating demand for disposable plastic blood bags.
Global demand for disposable plastic blood bags is expected to grow continuously with improvement in product features like value-added blood collection bags with in-line leucocyte filtration system, customized blood bags with tracking systems etc.
Manufacturers are competing on the basis of pricing to secure contracts from government organizations. Blood bag manufacturers in Asia-Pacific are majorly focused toward exports due to significant opportunity in European countries. Suppliers and distributors dealing in medical consumable supplies are targeting private hospitals, clinics, and blood collection centers for offering blood bags. However, the distribution margin for suppliers in private sales tends to be low, owing to lower volume purchases.
The most unprecedented operational and economic challenges followed the onset of the global COVID-19 pandemic. Also, considerable time is needed for the development and commercialization of effective treatment options for new pathogens.
In addition, local government redirection of healthcare resources toward COVID-19 treatment is also expected to engage most healthcare workers to tackle the outbreak. This uncertainty is expected to delay and reschedule the diagnosis and treatment of blood-related health conditions for the coming two to three quarters.
The blood bags market is expected to pick pace post-pandemic in the second-half of 2021, owing to the availability of treatment and recovery of developed and emerging economies from the COVID-19 pandemic.
Blood collection tubes. The blood collection tube segment is also anticipated to witness lucrative growth during 2020-2027. This can be attributed to the increase in the incidence of diseases that drives the demand for various blood testing methods, which in turn is fueling the segment’s growth. Moreover, ongoing technological advancement is another factor that is expected to fuel market growth. For instance, Becton, Dickinson, and Company’s Vacutainer Barricor, a plasma blood collection tube, has advanced technology that helps in blood collection and separation which offers improved clinical efficiency, sample quality, improved patient care, and faster test results.
Key drivers
The burden of chronic diseases is rapidly increasing worldwide. Almost half of the total chronic disease deaths are attributable to cardiovascular disease (CVD). Obesity and diabetes are also showing worrying trends, not only because they already affect a large proportion of the population but also because they have started to appear earlier in life.
The prevalence of lifestyle diseases is also growing across the globe, and particularly in emerging countries. This scenario has ensured greater adherence to health checkups and the growing importance of markers to find disease conditions mainly via blood collection. Health checkups are gaining popularity both at a personal level as well as performed at a corporate level for employee well-being. This will be favorable for the market growth and a significant contributor to the blood collection devices market, as blood tests are a primary mode of diagnosing these diseases.
Restraints
For blood banks, capital investments in automated blood collection using apheresis devices are very high in comparison to that of whole-blood collection. The average price for devices ranges between USD 45,000 and USD 55,000.
Hence, smaller blood banks and hospitals do not opt for this type of blood collection. The cost of therapeutic apheresis is also very high, as it includes the additional cost of disposables—between USD 1500 and USD 3000 per patient. Owing to their high costs, the adoption of these automated blood collection products is very low as compared to manual blood collection products in countries such as China and India. This is limiting the overall growth of the blood collection devices market.
The cost of providing apheresis therapy is a matter of almost universal concern.
Complexities of storage and shipping
Storing and shipping whole blood samples poses significant challenges and costs. Once collected, whole blood must be used immediately or stored and maintained under strict temperature and environmental conditions for analysis or other applications.
The characteristics of blood samples begin to change within hours of collection if not refrigerated or frozen. Maintaining their stability is essential since blood components begin to degrade immediately; extended exposure to ambient temperatures can dramatically affect the outcome of any analysis on such samples. Therefore, medical organizations around the world have created guidelines for their storage, packaging, and shipping.
For example, the US FDA recommends that whole blood samples held in specialized containers should only be kept refrigerated for 42 days. However, it is recognized that some changes in the samples may occur during that time. Another governing body, the National Institute of Health and Welfare in Finland, determined that whole blood samples refrigerated at 4°C (or about 39°F) should only be held for up to seven days before discarding.
Opportunity
Collecting blood samples from patients with difficult venous access (DVA) is challenging or sometimes impossible. In DVA patients, traditionally used blood collection products are often unable to collect adequate samples, which can also lead to repeated attempts to collect blood. This increases the risk of anemia in patients and the risk of transmission of blood-borne pathogens to nurses and phlebotomists.
To overcome this issue, innovative hematology-tube designs have been introduced to support capillary-blood collection for reducing the risks of collection and processing errors in DVA patients. Besides this, a vein illumination and visualization technique—vein finder, a recent addition to safe blood collection procedures—is used to assist healthcare professionals in finding a good vein for venipuncture.
The device illuminates the veins beneath the skin using ultrasound or infrared technology and facilitates easy vein access, thus reducing the need for repeated venipuncture.












