HPLC Systems
Unveiling Advances in Liquid Chromatography

From liquid chromatography, the technology advanced to high-performance LC and then to ultra-HPLC. Despite being the newest form of LC, UHPLC platforms come in many forms.
Many advances have been made in the world of liquid chromatography (LC) since its introduction over a century ago by Mikhail Tsvet. Obviously, the creative works of many scientists contributed to the development of LC, especially from the 1940s to the 1980s. However, central to the widespread development of the technique was the development of practical instrumentation by Csaba Horváth and Sandy Lipsky at Yale University in the mid-1960s to mid-1970s to create what is today known as high performance LC (HPLC).
Nearly five decades on, HPLC has become a big business. Market research firm, Top Down Analytics, projected 2018 worldwide HPLC instrument revenues of over USD 5 billion, with daily usage across such industries as pharmaceuticals, food and beverages, cosmetics, environmental safety, forensic medicine, and many others. Without the widespread deployment of HPLC, one would be faced with a world where many items would be less safe for human consumption, protecting the environment would be more difficult, and accurately identifying certain criminal activities would be nearly impossible.
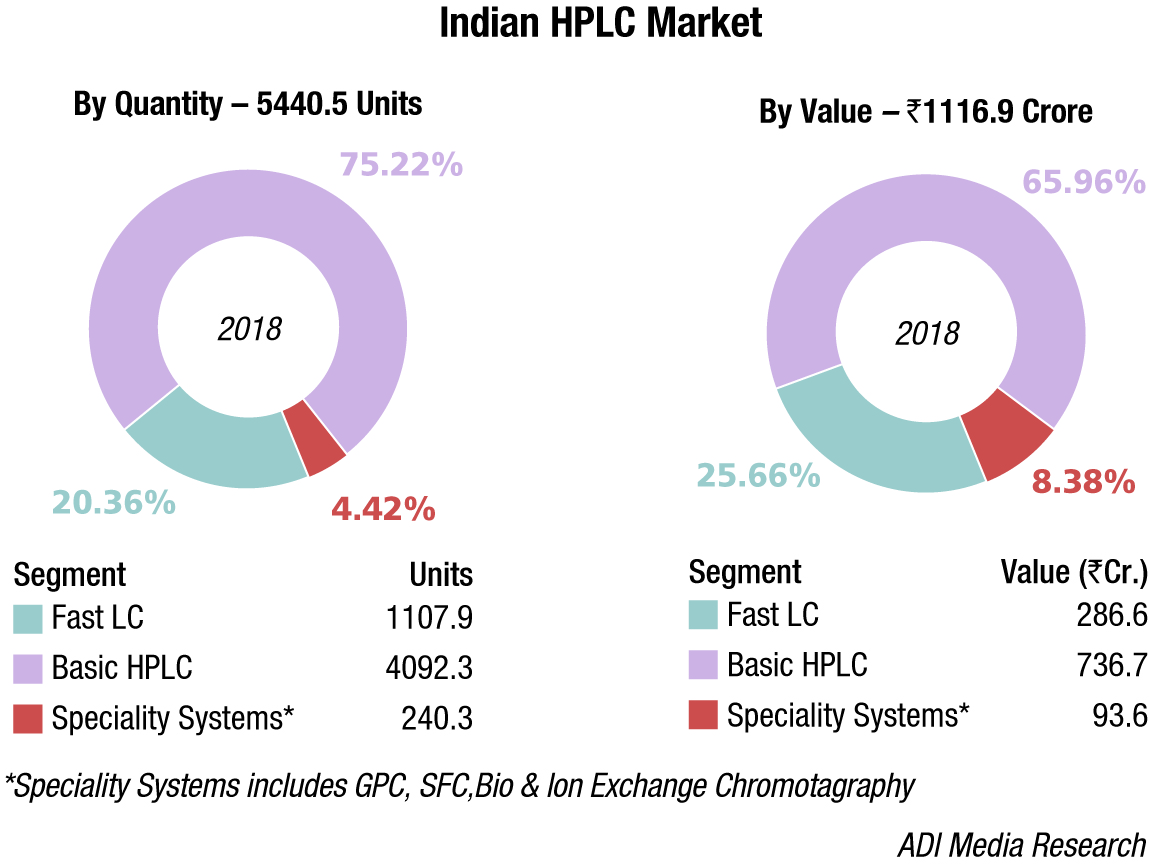
Indian market
The Indian HPLC systems market saw a decline of 10 percent in 2018, or was at the best flat for some vendors. This may be attributed largely to a dip in the market from non-compliance.
After years of struggle, the Indian pharmaceutical industry finally got many things right in 2018 with the American government’s drugs regulator. Not only did inspection outcomes improve, far more in line with global outcomes. The number of cases where the Food and Drug Administration (FDA) there chose to classify a production unit here under the Official Action Indicated (OAI) category fell sharply.
From 27 cases of OAI in 2014 and 22 in 2017, the number shrank significantly to seven. After an inspection, the FDA classifies a plant as either OAI, VAI (Voluntary Action Needed) or NAI (No Action Needed).
This change came even as FDA-registered drug facilities rose 63 per cent in India between 2011 and 2018. In comparison, there was a 51 per cent increase in China, of 25 percent in the European Union and a 10 percent decline in the US itself.
Indian Pharmaceutical Alliance (IPA), the industry body that represents research-based companies here, had started an initiative around 3 years earlier to improve quality standards among members. In 2016 six IPA members – Cadila Healthcare, Cipla, Dr Reddy’s Laboratories, Lupin, Sun Pharmaceutical and Torrent Pharmaceuticals – set up a forum to address the issues of data integrity and quality culture.
The effort seems to be now bearing fruit. A recent study by IPA and consultancy firm McKinsey showed India had six per cent of FDA global inspections in 2014. This steadily rose to 14 per cent of global inspections in 2018. Of the total of 174 such inspections in India last year, only seven were classified as OAI; 91 were VAI and 76 as NAI. By contrast, in 2017, it was 22 OAI, 80 VAI and 43 NAI. There has been a reduction in data reliability, and investigation & root cause assessment related errors, noted the study. It says gaps in manufacturing systems and laboratory controls are now a leading source of non-compliance. Companies are increasingly investing on improving such compliance. The industry is adopting more of automation and electronic data management and have a better understanding on compliance issues.
The HPLC systems market is expected to pick up once again by 2020.
Vendors update
Agilent 1260 Infinity II Prime LC systems. The family now includes binary pumps (600-bar) and 800-bar flexible pumps in the 1260 Infinity II Prime LC systems. Agilent Intelligent System Emulation Technology (ISET) removes method transfer challenges through a single click of the mouse.
| Tier I | Tier II | Tier III | Others |
|---|---|---|---|
| Waters | Agilent, and Shimadzu | Thermo Fisher (includes Dynex) | Yangling, Perkin Elmer, Hitachi, Knauer, and Tosoh |
| *Vendors are placed in different tiers on the basis of their sales contribution to the overall revenues of the Indian HPLC systems market. | |||
| ADI Media Research | |||
Sciex Jasper HPLC system. Jasper is a US Food and Drug Administration (FDA) Class I and CE-marked in vitro diagnostic system and serves as the front-end to Sciex’s MS systems and is designed for targeting applications in clinical diagnostics. They are designed to operate at a maximum pressure of 10,000 psi.
Thermo Scientific Vanquish UHPLC systems. The company updated its family of Vanquish UHPLC systems, now consisting of Vanquish Horizon (1500 bar, binary pumps), Vanquish Flex Binary (1000 bar), Vanquish Flex Quaternary (1000 bar, low-pressure mixing), and Vanquish Duo (dual pumps, two flow paths, up to 1500 bar, and dual split sampler) systems for enhanced productivity.
Waters Arc Bio system. The system is a dual-path, biocompatible, intermediary-pressure (9500 psi, 5.0 mL/min) UHPLC system designed to facilitate method conversion between UHPLC and HPLC. The system has an iron-free sample flow path and is particularly suited for the analysis of biomolecules, and is controlled by the Waters Empower 3 CDS or Masslynx software.
Bruker nanoElute UHPLC system. The company introduced the nanoElute UHPLC system for its Q-TOF and other MS systems in omics, biomarker, and biopharmaceutical applications. The system is equipped with a single-piston pump of 1300-µL volume, which supports flow rates of 50-2000 nL/min at pressures as high as 1000 bar.
Buyers’ perspective
While procuring an HPLC system, buyers take into consideration:
- Flexibility of the system – whether the system can be optimized to meet the laboratory requirements;
- If components, such as additional detectors and valves, can be upgraded in the future;
- They ask for a demo version, to get a feel of how the software functions for the laboratory’s workflow;
- If the system (not just components) is qualified during installation as to meeting the manufacturer’s performance expectations;
- Ensure after-sales service is available, whether from the manufacturer or from a third-party service group, and if the service providers are factory-trained; and
- Finally, check on the cost of the purchase – not just the price of the product being installed but the total cost of ownership, which includes price, service expectations, and warranty, among others.
Way forward
The ever-increasing demands on liquid chromatography have fueled ongoing improvements and enhancements of the technique to provide better and faster separations. With the need to achieve complete separation of more and more complex compounds with greater precision, UHPLC has become a standard practice across numerous disciplines.
Industry Speak
Current Trends in Diagnostics
Shailesh Damale
Assistant Manager
Shimadzu Analytical (India) Pvt. Ltd.
Many forms of chromatography have been used over the years in the clinical laboratory for the separation and quantification of a variety of clinically relevant analytes. The main advantages of HPLC method over other techniques are their high selectivity, sensitivity, reliability, versatility with low cost of operations. Its use in the clinical laboratory has steadily increased over the past decades as its unmatched analytical performance and versatility allows for testing of many different types of clinically relevant analytes.
Medical diagnosis should be fast, reliable, specific, accurate, and should minimize possibility of false positive. Diagnostics tools and methods with high degree of sensitivity and specificity aid in early detection of diseases and disorders; and hence can provide better prognosis. Cutting edge technology for medical diagnosis is very vital as it directly affects healthcare of the general population.
India is an ethnically diverse country with marked regional variations. This diversity is reflected in the presence of different hemoglobin variants in different ethnic groups. Due to migration, there is constant mixing of people from different regions. Many of these abnormal variants are of little clinical significance in heterozygous state, but when combined with other variants they may give rise to several severe diseases. Therefore, there is always need for a screening method that can detect maximum variants. HPLC has the advantage of quantifying Hb F and Hb A2 along with the detection of other variants in a single screening test.
HPLC being a versatile tool, has been used for low level quantitation of vitamins, nucleic acids, biogenic amines, TDM like, immunosuppressant, and so many. HPLC-based methods would remain the gold standard in clinical testing for many of the current as also for future biomarkers and therapeutic drugs.
Because of government initiatives and strict regulations, testing labs are forced to go for more selective, precise and automated tools like HPLC that would find central place in any diagnostic lab.
Such advancements and benefits notwithstanding, the reality is that there are multiple limiting factors to the vast majority of HPLC systems deployed today. Examples of such factors include size and weight per unit (upwards of 24 cubic feet and 100 pounds each), as well as the ongoing costs of solvents, waste disposal, and environmental risks.
So, what happens to the world of analytical chemistry when HPLC instruments become small and portable?
The obvious benefits of shrinking
As it turns out, there is little available information on the internet today that discloses the exact dimensions or weights of the most widely deployed HPLC instruments, let alone the volumes (or costs) of solvents each consumes in round-the-clock usage, or the waste volume requirements for HPLC use, either on a storage or a disposal basis. But, from a practical standpoint, even if they are not responsible for the financial aspects of HPLC use, one can clearly understand how large and heavy most HPLC instruments are; the average number of liters of solvents consumed per month per standard HPLC system; and the waste storage and disposal requirements for using today’s HPLC instruments.
So, what happens if one shrinks HPLC systems? At a minimum, there are at least four obvious benefits from making HPLC instruments smaller:
Smaller means less space. As soon as HPLC systems shrink in size, they take up less space, whether in a laboratory setting (such as on a laboratory bench or within a fume hood) or on a factory floor. At a minimum, a smaller footprint opens up real estate for other instruments, equipment, or supplies wherever typical HPLC instruments have been deployed previously.
Lower solvent use. Smaller HPLC instruments become practical only if they utilize capillary columns within a high-pressure system, and, when designed properly, HPLC systems with capillary columns will mean lower solvent and sample usage.
Smaller waste volumes. If solvent and sample needs decrease with smaller HPLC systems, then, ipso facto, waste volumes will drop as well.
Decreased costs. When solvent consumption drops, and the amount of waste experts are producing also drops, costs should drop in lockstep. Specifically, experts will spend less on solvents, because they will need lower amounts per analysis. Additionally, because they will be producing less waste per month, their waste disposal costs will also drop.
Not-so-obvious benefits of shrinking
In-laboratory portability and mobility. The moment HPLC instruments weigh less and take up less space, it becomes immediately possible to move an HPLC system from one location within a laboratory to another, without a forklift, and fairly easily and safely as well. Depending on the design parameters of the respective mobile HPLC instrument, it might be necessary to recalibrate a system after it has been moved, but that is the case already today.
Manufacturing portability and mobility. If smaller and lighter-weight HPLC instruments can be easily moved around within a laboratory setting, similar portability and mobility benefits are also available within manufacturing facilities as well.
Eradicate jury-rigged mobile platforms. It has been observed that a variety of efforts designed to transform bulky HPLC systems into mobile solutions are used on manufacturing lines, and implemented for transportation between laboratories. The most common example has been the use of industrial carts laden with HPLC instruments, liters of solvents, and waste capture containers. Ingenious as such hand-pushed contraptions may be, they are, in reality, inherently unsafe and cumbersome attempts to solve important analytical challenges.
Eliminate Rube Goldberg-like laboratory setups. So, what happens when an HPLC instrument is located on a bench at one end of the lab and the mass spectrometer (MS) is at the other end of the lab, and one needs to get a sample from their instrument to the MS instrument? If the MS instrument is not dedicated to an HPLC system, the HPLC instrument must be moved onto a cart, and wheeled as close to the MS system as possible, relying on transfer tubing to make the final connection, with its related delay volume and other challenges. But, with a hand-portable, shrunken HPLC instrument in play, the need for such Rube Goldberg-like setups is eliminated, permanently.
Moore’s Law and dedicated HPLC systems. When Gordon Moore first posited what became known as Moore’s law, few anticipated the decades-long implications tied to ever-shrinking semiconductors, the doubling of computing power, and the attendant price drops of such processing chips. Interestingly, other industries have experienced similar growth curves often tied to smaller components, increased capabilities, and lowered prices. As HPLC systems shrink and their attendant costs drop, both from a capital expenditures standpoint and from an operational expenditures perspective, one potential benefit of such a world is the ability for organizations to move to dedicated HPLC instruments for their scientists, instead of shared systems, and, if more analytical chemists have their own HPLC systems, the opportunity for increased throughput rises significantly.
In addition to the outcomes outlined, the most intriguing potential benefits of smaller and lighter-weight HPLC instruments are those that experts do not know yet, or those that they have yet to discover. Perhaps, these are what one might consider as the unintended consequences of shrunken HPLC systems. Be that as it may, the most unexpected opportunities of smaller HPLC systems will be the ability to take the laboratory to the sample. Other potential unexpected applications for shrunken HPLC systems might include: crime scene HPLC instrument deployments; real-time drug testing for employers; or on-the-fly environmental testing of chemical spills, just to name a few.
Second Opinion
HPLC, Breaking Though Traditional Barriers
Dr Jyoti Upadhyay
Assistant Professor
School of Life Sciences
High Performance Liquid Chromatography (HPLC) is an important chromatographic technique based on the separation of molecules because of the differences in their structure and composition. The separation technique depends upon the overall interactive relationships between analyte, mobile phase, and stationary phase. It is generally improved form of column chromatography and offers safest, fastest, automated, and accurate method for identification of certain components in a sample.
Column switching. If the flow through sequence is branched and switching devices are employed to interface individual columns, exerting powerful control on the quality and the basis for the separation, this versatile nature makes liquid chromatography a powerful separation system. It leads to the idea of column switching, means that the carrier flow is changed by standard six-port high pressure valves, as a result a fraction of or all of the effluent from any column (primary) is transferred to other column (secondary) forming a network for further separation.
In modern liquid chromatographic techniques, the term column switching is used for different operating modes in a strictly non-defined sense. Column switching can also be synonymously expressed as sequential chromatography, coupled-column chromatography, multi-channel chromatography, multiple chromatography, split chromatography, but they are used with different objectives for chromatographic separations. Multi-dimensional liquid chromatography. It provides multiple dimensions based upon different separation mechanism having sufficient resolving power for the separation of complex mixture in food analysis, molecular biology, metabolomics and proteomics.
Micro-column and nano-liquid chromatography. For carrying out technological, theoretical, and methodological studies, especially in the field of analytical chemistry miniaturization of liquid chromatographic instrument is done. This provides shorter analysis time due to lower flow rates, better separation efficiencies and successive coupling with MS.
Chip-based liquid chromatography. Micro-fabricated devices (named μ-total analysis system, μ-TAS) are used for analyzing minute samples in the shortest possible time and integrating in the same instrument both pretreatment of the sample and separation. The main advantages of these devices are the low LODs (Limit of detection), increased sample loading and peak capacity and the minimization of handling and transfer steps they offer.
Ultra-performance liquid chromatography (UPLC). It is new direction of liquid chromatography. UPLC technique has made progress in particle chemistry performance, detector design, system optimization, data processing and control.












