Flow Cytometers
Flow cytometry gaining recognition
As flow cytometry is being increasingly used in new applications, particularly nontraditional immunology workflows, the technique must become less complex, and this will happen by making instruments smarter and preconfiguring the reagents.
Flow cytometers have now become essential instruments in biomedical research and routine clinical tests for disease diagnosis, prognosis, and treatment monitoring. Recently, use of flow cytometry in cancer immunology has grown impressively, because of the role it can play in extending the understanding of immune system and its response to cancer immunotherapies. For such a familiar technique, flow cytometry holds many surprises. It is more compact, more maneuverable, and more available than ever. Consequently, flow cytometry is interjecting itself where it was once unknown or where it is still considered nontraditional. For example, flow cytometry is getting closer to clinicians, environmental field workers, and food-safety inspectors. Flow cytometry is also finding new uses in familiar settings, such as the research laboratory and the biopharmaceutical production line.
Long capable of running phenotypic screens, flow cytometry is starting to apply its powers of discrimination to genetically modified cells. Flow cytometry is validating transfection, confirming whether desirable edits have been achieved, measuring the functional effects of gene editing, and enriching cell populations based on functional cell sorting.
Compatible with auto-sampling and high-throughput technology, sensitive to a rainbow of colors, capable of processing multiple inputs in parallel, and reconcilable with activities upstream and downstream (including PCR analysis and genome sequencing), flow cytometry is helping advanced single-cell analysis. While the combination of flow cytometry and single-cell technology is still new, it is already defining new developmental states, identifying unculturable microbes, and revealing how cellular heterogeneity relates to health and disease.
Clinical uses of flow cytometry, which has exploded in recent decades, include validating hematopoietic stem cells pre-transplantation and identifying irregularities in immune cell subsets that are present in cancer and immunodeficiency disorders. As instruments have become smaller and easier to maneuver, flow cytometers have also come to be used for near-patient disease diagnosis.
Additionally, scientists are adapting instruments for nontraditional flow cytometry applications, from biomanufacturing and bioprocessing monitoring (including water-quality testing and agricultural and food-safety certification) to veterinary medicine, oceanography, and ecological field research. Given their high-throughput capacity for detecting and quantifying analytes in solutions and given their multiplexing potential, flow cytometry systems will be quickly adapted to diverse research and industrial sectors.
Looking forward, experts see an increasing need to democratize flow cytometry. Today, it is still considered a highly complex technique, requiring an expert operator and a time-consuming experimental design process. As flow cytometry is increasingly used in new applications, particularly nontraditional immunology workflows, the technique must become less complex, and this will happen by making instruments smarter and preconfiguring reagents.
Technological advancements
Most advances in flow cytometers have depended on co-creation, with scientists, research institutions, and companies working together. In recent years, researchers are paying increasing attention to the development of portable microfluidic diagnostic devices, including microfluidic flow cytometers for point-of-care (PoC) testing. Microfluidic flow cytometers, where microfluidics and flow cytometry work together to realize novel functionalities on the microchip, provide a powerful tool for measuring the multiple characteristics of biological samples. The development of a portable, lowcost, and compact flow cytometer can benefit the healthcare in underserved areas.
CRISPR technology. In the past few years, the market has seen an explosion of companies utilizing flow cytometer in the CRISPR process, doing everything from determining transfection rates to sorting cells. These activities are being facilitated by flow cytometers. Manufacturers have incorporated flow cytometry into the CRISPR workflows. As a CRISPR tool, flow cytometry is important not only for validating the correct targeting performed by CRISPR, but also for measuring the functional effects of gene editing. By pairing multiple tools and techniques, new systems have increased workflow efficiency. At the end of the day, scientists using CRISPR want to know whether they have successfully edited their target cells before moving to downstream assays. Flow cytometry and functional cell sorting will be increasingly important technology to help answer several questions as the field of genome editing evolves.
Integration with MS. Recently, a new analytical approach, called mass cytometry, which combines the precision of mass spectrometers with the power of flow-cytometric analysis, has been developed. The application of both techniques in the field of cancer immunotherapy is very promising, and a number of related applications are under development. If implemented, this may allow for the generation of large amounts of multidimensional data that are amenable to high through-put analysis, and this may offer unique opportunities and challenges for the field of biomarker development. The use of these powerful technologies will answer a multitude of questions with just one sample being analyzed, and this will likely help to tailor cancer immunotherapies to each patient. However, at the moment, the quantity and complexity of data obtained with this technology requires some analytical considerations.
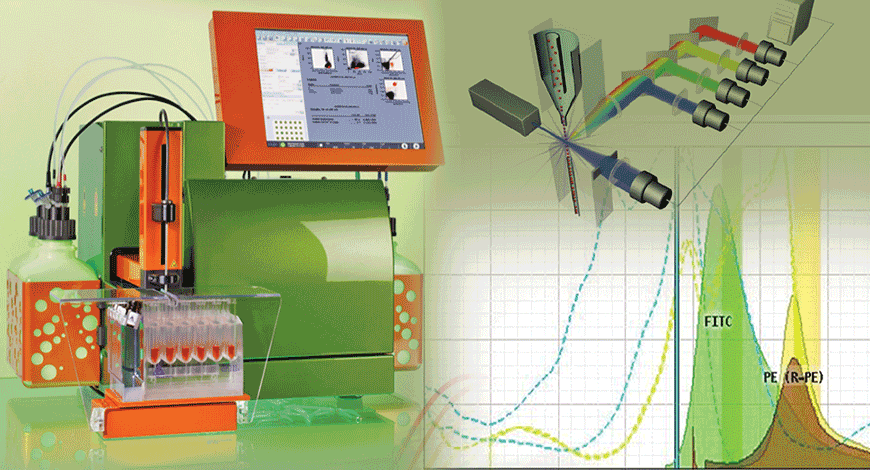
Adding spectroscopy to flow cytometers. Aided by recent innovations and advances in semiconductor detectors, telecom optics, and computation that enable sub-millisecond, full spectral measurements, spectral (or multi- or hyperspectral) flow cytometry has emerged in recent years and is now being adopted by a wider group of scientists. Spectral flow cytometers, which incorporate ultrafast optical spectroscopy, have simpler optical paths and fewer components than conventional flow cytometers, and they provide higher-quality results with fewer lasers. A major feature of spectral flow cytometry is, of course, spectral unmixing – that is, analysis of the spectral data to extract revealing information. Spectral unmixing provides accurate estimates of label intensity and resolve fluorochromes with significant spectral overlap. The algorithm used for this process replaces the compensation matrices of conventional flow cytometry and treats autofluorescence as an independent parameter. All of this adds up to less-complex, more-capable instrumentation as well as greater flexibility, as researchers get much more choice in designing assays.
PoC microfluidic flow cytometry. In recent years, research is focused on developing microfluidic flow cytometers with the motivation of creating smaller, less expensive, simpler, and more autonomous alternatives to conventional flow cytometers. These devices could ideally be highly portable, easy to operate without extensive user training, and utilized for research purposes and/or PoC diagnostics, especially in limited-resource facilities or locations requiring on-site analyses. Microflow cytometers that combine microfluidics and miniaturized detection systems are a promising solution for PoC diagnosis. The recent innovations in particle focusing and detection strategies are used to fulfill the criteria of high-throughput analysis, automation, and portability, while not sacrificing performance. The ongoing contribution of microfluidics demonstrates that it is a viable technology to advance the current state of flow cytometry and develop automated, easy to operate, and cost-effective flow cytometers.
Large particle analysis. Addressing the problems inherent to large particle analysis and sorting, manufacturers now offer a range of flow cytometers that have been optimized for large cells, cell clusters, small model organisms, hydrogel encapsulated particles, organoids, and organotypic tissue fragments. While the principles of flow cytometry are essentially the same, larger flow cells work at lower pressures than conventional flow cytometers to reduce shear forces and provide gentler sample handling. Larger passage through the flow cell affects the fluidics – a factor that manufacturers have addressed thoroughly within the different instrument platforms. Also, the sorting mechanism is very different than conventional flow sorting, involving a gentle air diversion mechanism.
Way forward
The frontier of flow cytometry applications is expanding. Which role mass cytometry will play in all of this, remains open; the underlying technology is entirely different, as it is based on elemental mass spectrometry. For utilizing this way of detection, cells are tagged with heavy-metal isotopes instead of fluorescent dyes, thereby eliminating the problem of spectral overlaps. Clearly, there are inherent analytical advantages in terms of accuracy and signal-to-noise ratio, but instrument purchase costs are extremely high and the data output per experiment is difficult to analyze quickly.
Another technology potentially competing with flow cytometry is known as chipcytometry. Using up to 100 markers, it allows specimens to be phenotyped and banked on the same microfluidic chip, because tissue and cell suspension samples can be preserved for reanalysis after being stored for months. It is always daring to envision what the future holds, to predict which tools will become important or not. Although being challenged by the alternative platforms mentioned, still flow cytometry will not become obsolete and phased out from applied research. A lot will depend on how the technology can advance to become more quantitative. Ultimately, the more accurately measurements by flow cytometry can be performed, the more valuable its results can be for the users relying on them, may it be a clinician, a drug developer, or a food safety technician. Going forward, it will be essential that datasets can be considered valid, that is fully reproducible, irrespective of who, where, and on which bench-top flow cytometer these were generated.












