MB Stories
Impact of modernization of electrophoresis

As modernization continues to transform the field in 2023, the advancements are leading to an increased use of electrophoresis in clinical and diagnostic applications, where speed and accuracy are crucial.
A hundred years of electrophoresis innovation has finally landed in the cloud, from the first room-sized apparatus to today’s next-gen, toaster-sized, all-in-one device. The evolution of electrophoresis systems has come a long way, starting from bulky and intricate instruments that occupied entire rooms, to the modern compact and intelligent devices, capable of handling high-volume research. Despite advanced features, the design of the electrophoresis systems is simple and secure.
Electrophoresis has undergone modernization through the development of novel techniques, such as capillary electrophoresis, microchip electrophoresis, and high-throughput gel electrophoresis, resulting in improved analysis resolution, sensitivity, and speed. Moreover, the advent of automated systems has made electrophoresis more user-friendly and accessible, leading to its increased use in clinical and diagnostic applications that require rapid and precise analysis.
The modernization of electrophoresis has also led to the emergence of new applications in proteomics, genomics, and metabolomics, enabling researchers to analyze complex biological samples and gain a deeper understanding of cellular processes and disease mechanisms.
Overall, the modernization of electrophoresis has significantly impacted the field of molecular biology, providing researchers with powerful tools to investigate biological molecules accurately. As technological advancements continue, further improvements in electrophoresis techniques are expected, leading to new discoveries and applications.
Electrophoresis continues to be a widely used technique in proteomic and genomic research, disease diagnosis, and drug quality control. The market is expanding owing to rising funding for life science research, increased demand for proteomics and genomics research, and growing adoption of electrophoresis techniques in clinical settings.
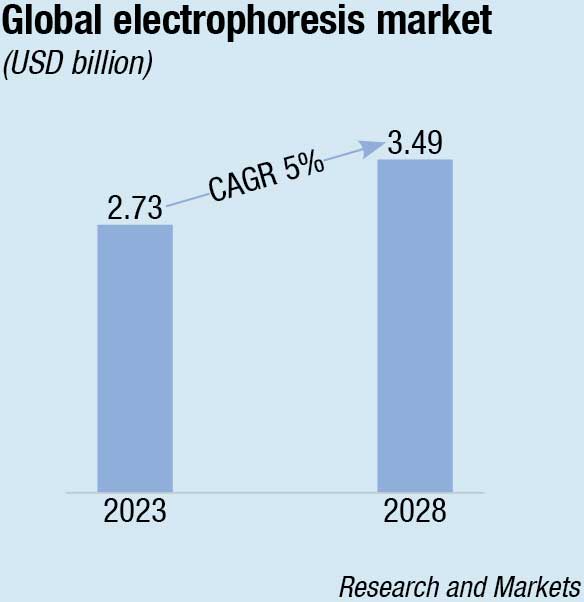
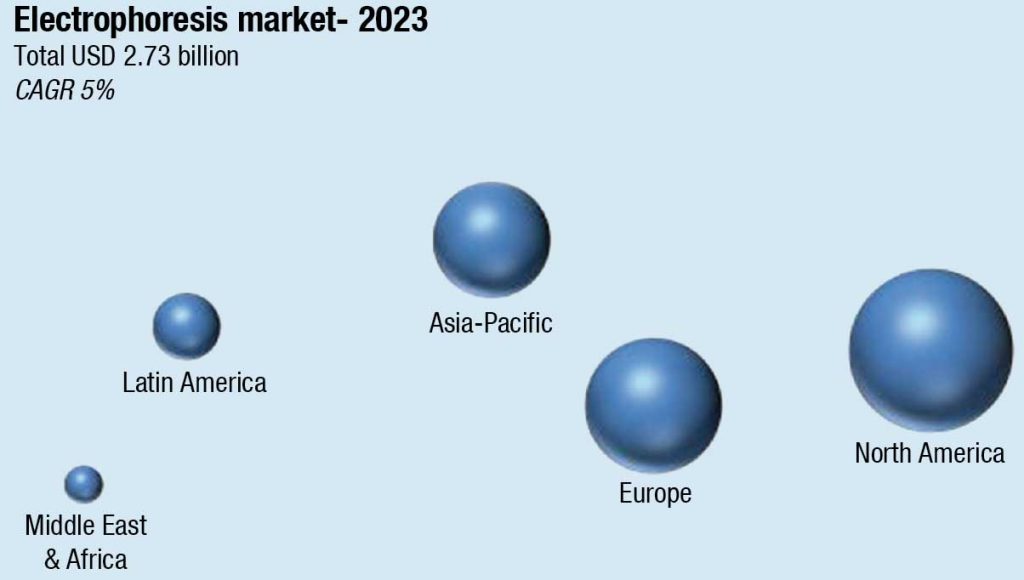
The global electrophoresis market in terms of revenue is estimated at USD 2.73 billion in 2023, and is poised to grow to USD 3.49 billion, at a CAGR of 5 percent from 2023 to 2028, according to Research and Markets.
Market players are collaborating with academic institutions to increase awareness of new analytical technologies among students and researchers. In August 2022, SciGenom Labs, India, and CHARUSAT University, USA, launched a unique Ph.D. program to support genomics, proteomics, microbiome disciplines, and bio-nanotech research. Numerous collaborative research projects have been launched, and more are expected. Companies are also providing technical and financial assistance to university researchers to help commercialize their research projects. These collaborations are expected to positively impact the electrophoresis market.
Furthermore, personalized medicine has become a core area of research in the healthcare industry, entered mainstream clinical practice, and is changing how diseases are identified, classified, and treated. This trend is particularly evident in oncology. This growing demand for personalized medicine is expected to increase the uptake of electrophoretic systems in the drug-development process.
The electrophoresis market in the Asia-Pacific region is growing rapidly due to the increased use of next-generation sequencing, research-based collaborations between academic and pharmaceutical industries, and increased diagnostic applications. Electrophoretic techniques are being widely used in clinical diagnostic testing, including the evaluation of protein expression profiles and disease-specific biomarkers for disease identification. Applications include hemoglobin testing, enzyme measurements, plasma, serum, urine, cerebrospinal fluid, multiple myeloma testing, lipoprotein, and hormone measurements. This trend is expected to contribute to the market’s rapid growth.
Prominent players in the electroph-oresis market include well-established, financially stable manufacturers of electrophoresis systems, reagents, gel-documentation systems, and software. Bio-Rad Laboratories, Inc., Thermo Fisher Scientific Inc., GE Healthcare, Danaher Corporation, Merck Millipore, and Agilent Technologies, Inc., PerkinElmer Inc., QIAGEN NV, Lonza Group, Shimadzu Corporation, Harvard Bioscience, Sebia Group, and CBS Scientific are some leading players.
HOEFER INC., 4Basebio PLC, Takara Bio Inc., Helena Laboratories Corporation, Syngene, VWR International, TBG Diagnostics Ltd., Analytik Jena GMBH, Oprl Biosciences, Kaneka Eurogentec S.A., Cleaver Scientific Ltd., Major Science Co. Ltd., Bio World, National Analytical Corporation, and AES Life Sciences are also aggressive.
Electrophoresis requires a complex combination of analytical knowledge, extensive manual handling, dexterity, and strict protocol adherence to achieve reliable results. Furthermore, the gel concentration must be accurate to avoid errors; if too high or too low, the fragments will migrate too slowly or quickly, leading to errors in resolving the different bands. Steady voltage is also imperative, as fluctuation results in unsteady migration of DNA fragments, leading to errors in reading the bands. The buffer solution must also be of the correct composition, as a buffer with the wrong pH or iconic concentration will change the DNA fragment shape and migration times.
Capillary electrophoresis mass spectrometry (CE-MS), a recently developed analytical technique, combines the high-resolution separation capabilities of capillary electrophoresis (CE) with the sensitive and selective detection capabilities of mass spectrometry (MS). This method allows for the detection of analytes across a wide range of sizes, from small molecules to large biomolecules. A volatile buffer solution is typically used to prevent the interference of salts with ionization during MS detection.
CE with MS is a promising technique for the analysis of complex traditional Chinese medicines (TCMs) due to its high sensitivity and efficiency. It can provide structural information and measure molecular weights of compounds in TCMs. MCE-MS and monolithic CEC-MS are considered green approaches as they require less reagents. However, the limited availability of dedicated CE-MS instruments is a challenge. The development of cost-effective, reliable CE-MS systems with necessary features, such as thermostat cooling, solvent gradient capability, and an autosampler is crucial for wider implementation of this technique.
Capillary electrophoresis (CE) could see increased usage in proteomics due to technological developments and changes in research trends. CE is a molecule separation technique that separates molecules based on their charge. Although CE has potential advantages over liquid chromatography (LC), technical challenges, such as small loading capacities have limited its usage in proteomics. However, firms like 908 Devices and CMP Scientific are addressing these challenges with on-chip sample pre-concentration and increasing sample loading capacities.
Capillary zone electrophoresis (CZE) with ultraviolet (UV) detection, a capillary electromigration technique, is frequently utilized for the efficient separation, identification, and quantitation of various active components in highly complex matrices.
Microchip electrophoresis (ME) continues to be popular for the rapid and cost-effective analysis of bacterial and viral pathogens in biological and environmental samples. However, accurate and sensitive analysis requires microbial enrichment and purification, which can be achieved through on-chip microfluidic-based sample preparation techniques, such as di-electrophoresis and microfluidic membrane filtration.
Progress has been made in the application of ME for the analysis of pathogenic bacteria and viruses. The prevalence of life-threatening diseases, including tuberculosis, pneumonia, hepatitis B, and Covid-19, has amplified the demand for sensitive, cost-effective, and rapid analytical methods for the detection of such microorganisms.
ME has gained considerable attention in recent years as it offers advantages, such as portability, simplicity, and rapid analysis, and is cost-effective. However, precise analysis of pathogenic bacteria and viruses in complex matrices necessitates critical steps, such as microbial enrichment and purification, but now advances made in sample preparation technologies, especially on-chip microfluidic-based sample preparations, such as di-electrophoresis and microfluidic membrane filtration, are associated with accurate analysis of these microorganisms.
Lab-on-a-chip electrophoretic analysis has seen advancements in recent years in the analysis of pathogenic bacteria and viruses in complex matrices. Integration of nucleic acid-based amplification techniques, such as PCR, qPCR, and multiplex PCR with ME has resulted in accurate and sensitive analysis of microbial pathogens, which is essential for point-of-care diagnosis of infectious diseases.
The microfluidic chips are a promising platform for performing isoelectric focusing (IEF) due to their high resolution, sensitivity, and reproducibility. The reduced dimensions of microfluidic channels enable faster separation times, lower sample volumes, and higher throughput compared to traditional IEF methods. Furthermore, microfluidic devices can be readily integrated with other analytical techniques, such as mass spectrometry, to improve the detection and identification of separated biomolecules. IEF has a wide range of applications, including the separation of proteins, antibodies, peptides, and other bioactive substances. Additionally, IEF has significant clinical applications, such as disease diagnosis and monitoring, as well as the development of novel therapeutic agents.
The microfluidic chip, a miniaturi-zed laboratory, integrates multiple analytical instruments into a single device. It contains microchannels that connect the sample wells to a separation channel and buffer wells. By applying a voltage across the microchannels, using an electrophoresis station, charged molecules in the samples move into and through the separation channel. The chip operates in a sequential mode, with a time delay between samples to avoid cross-contamination.
Microfluidic technology allows for lower consumption of sample and reagent quantities, and expedites the run and analysis time, relative to conventional analysis methods. By reducing hands-on time, microfluidic automated electrophoresis systems enable the completion of protein or nucleic acid electrophoresis and analysis of up to 12 samples in a single 30–40 minutes assay.
The automated electrophoresis systems are set to be positively impacted by the adoption of advanced technologies, driven by increased R&D spending and growing industry focus on innovation leading to novel breakthroughs. This will result in new players entering the market and increasing competition. Consumer demands will continue to evolve, which will be met by new product launches with advanced features or competitive pricing, as established companies aim to maintain their market leadership and new entrants try to establish their market presence. Automation in electrophoresis is making it a more efficient and less labor-intensive process, employing microfluidic chip-based technology that integrates electrophoretic separation, fluorescence detection, and data analysis of various biomolecules, such as proteins and nucleic acids, within a single platform.
Outlook
The modernization of electrophoresis has revolutionized the field of molecular biology by enabling researchers to analyze complex biological samples with improved analysis resolution, sensitivity, and speed. The evolution of electrophoresis systems and techniques is expected to lead to new discoveries and applications in the future.
And, with the Indian biotechnology industry poised to reach USD 150 billion by 2025, electrophoresis is set for growth.












