Company News
Inspira Technologies reveals next generation Liby ECMO
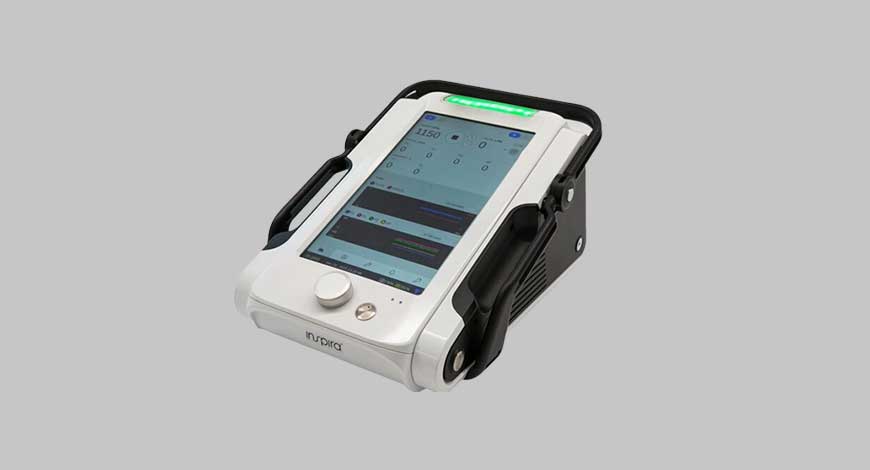
Inspira™ Technologies OXY B.H.N. Ltd. introduces today the “Liby™” System, an advanced form of life support better known by the medical industry as extracorporeal membrane oxygenation (ECMO), used to treat patients with life-threatening heart and lung failure. The Liby™ System is intended to target the $531million global ECMO market. The Inspira Technologies Liby™ system currently under development, is expected to be submitted to the U.S. Food and Drug Administration (FDA) for approval, during the first half of 2023.
The Inspira Technologies Liby™ system is designed to be a new generation ECMO system, with potential advantages that may improve usability and patient care. The Liby™ system is also expected to be the first system designed for integration with the Company’s recently revealed non-invasive HYLA™ blood sensor technology. The addition of the HYLA to patients treated with the Liby™ system, would potentially allow for the real-time and continuous monitoring of patient condition to alert physicians of immediate signs of changes in a patient’s clinical condition.
The Liby™ system includes several Inspira Technologies developed features and capabilities as well as a new approach to medical device designs, including a large touchscreen and novel colorful graphical representation that increases the visibility, scope and functionality of data displayed to the medical staff. With its small footprint and lightweight characteristics, the Liby™ system is being designed with a rapid style aerospace-grade aluminum structure to be both lightweight and highly durable, and will be equipped with long battery life, a contributing factor to making the Liby™ system suitable for patient mobility within hospitals and for transportation of patients to hospitals in ambulances.
Product launch
The Liby™ system is expected to be submitted to the FDA for approval during the first half of 2023. Subject to FDA approval, the Liby™ system’s expected regulatory pathway is intended to be designated as a Class II 510 (K), meaning it may not require human trials.
Business model – Recurring revenue
Similar to the Company’s flagship ART™ system, the Liby™ system is being designed to generate recurring revenues based upon consumable sales of its single-use disposable kit, which is an important part of the system’s unique design, aimed at reducing setup time and associated medical-care costs.
Dagi Ben-Noon, Inspira™ Technologies’ Chief Executive Officer, stated: “The Liby™ system is intended to target one of three market segments within Inspira Technologies product scope. The Liby™ system is expected to introduce the next generation of ECMO within Intensive Care Units (ICUs), due to its practical advantages, designed to improve durability, patient mobility both within the hospital or via ambulance, and ease of use. I believe that these are important advantages for medical teams within over-crowded ICUs, targeting to improve patient outcomes.”
MB Bureau












