Industry
RAT-A radical method for affordable and speedier COVID-19 diagnosis
Testing has been playing a pivotal role in tracking and management of global COVID-19 pandemic. The continuously increasing demand for reliable COVID-19 detection tests has encouraged the adoption of innovative and faster diagnostic methods.!
The SARS-CoV-2 infection started from a small province of China in March 2020 and has now spread to almost all countries of the world. The medical world is in an upheaval as the race for vaccine development continues. But, while the search for a vaccine is on a fast-track, the importance of reliable and affordable diagnostic testing services has not diminished.
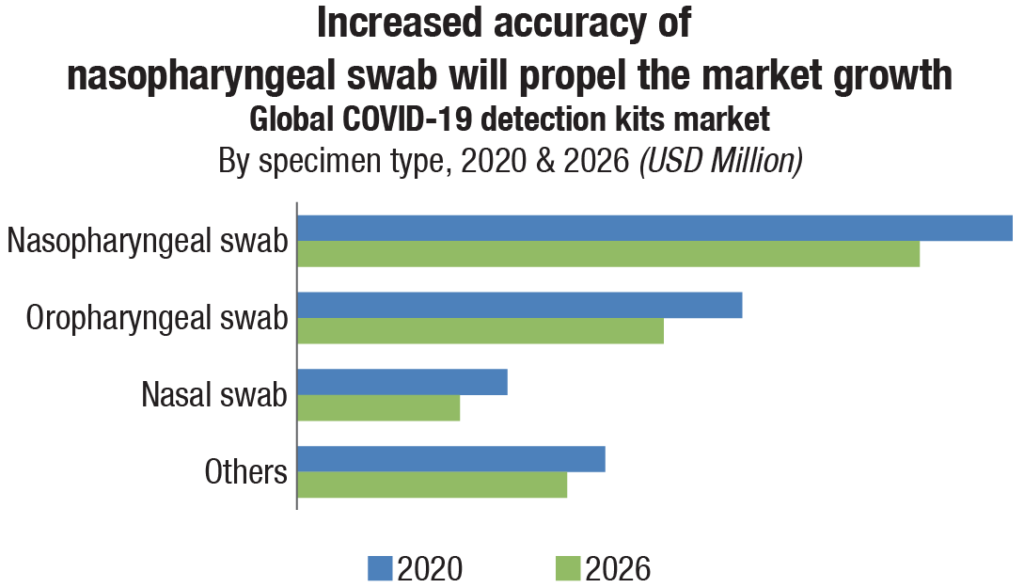
With the global upsurge in COVID-19 cases and mortality rate, a huge demand for affordable, simple, and fast result providing testing kits was created. The COVID-19 detection kits market for the year 2020 has been estimated at USD 15.4 billion, forecast to fall to USD 13 billion in 2026, a 2.8 percent compound annual growth rate (CAGR) decline. To meet the exigency, the forerunner manufacturers in the molecular diagnostic industry, such as Roche, Abbott, Qiagen, and BD, shifted their focus to development of rapid COVID-19 antigen testing kits.
The nasopharyngeal swab segment (encompassing PCR tests and antigen tests) accounts for more than 40 percent COVID-19 detection kits market share worldwide in 2020. COVID-19 is a respiratory disease, and therefore, most samples for detection tests are based on nasopharyngeal swab to provide high yield and specificity.
It has been widely recognized that there is a need for efficient and accurate COVID-19 diagnosis that is sensitive, portable, and enables on-site diagnostic testing, allowing real-time management of patients in minimal time. This has encouraged the use of point-of-care testing for screening susceptible COVID-19 cases, with a primary focus on reducing the waiting time for results from hours to minutes. Rapid Ag detection tests for COVID-19 detection remain a key contributor in screening and breaking the cycle of the infection transmission.
Food & Drug Administration (FDA), USA has granted emergency use authorization (EUA) for antigen tests to identify and diagnose SARS-CoV-2 in symptomatic and asymptomatic patients. Abbott’s sixth COVID-19 test, the BinaxNOW COVID-19 Ag Card rapid antigen test, received EUA from FDA. The test gives the result in 15 minutes which can be displayed on a new optional app called NAVICA (at no charge) on both iPhone and Android devices. Quidel, BD, and LumiraDx, UK too have received EUA. Quidel earlier reported the sensitivity of its test kits to be 96.7 percent and BD reported sensitivity of 84 percent. Both the companies now claim their tests to have a specificity of 100 percent.
The test developed by Roche in partnership with SD Biosensor Inc., has a sensitivity of 96.52 percent and specificity of 99.68 percent (426 samples from two independent study centers). The company claims to provide 40 million SARS-CoV-2 rapid tests per month, with more than two-fold increase in capacity at the end of this year to enable a fast mode for testing, tracing and treating people especially in areas with under-resourced health systems.
The tests are highly portable, reliable, and easy to use, facilitating testing in near-person, decentralized healthcare settings. Roche and SD Biosensor Inc. have a global distribution agreement.
Abbott and SD Biosensor formed an agreement with the Bill & Melinda Gates Foundation to help make these innovative tests available at price maximum of USD 5 for low- and middle-income countries (LMICs). The Global Fund committed an initial amount of USD 50 million to enable countries to purchase the new range of tests. The National Institutes of Health, USA, invested USD 248.7 million in new technologies to deal with the challenges associated with COVID-19 testing.
The test itself
The rapid antigen detection test for COVID-19 is a sensitive, point-of-care test that enables accurate screening of individuals with known exposure to SARS-CoV-2 virus. This nasopharyngel swab test is based on detection of proteins from the Covid-19 causing novel coronavirus in respiratory samples such as sputum, throat swab, etc.
 A typical antigen test starts with a healthcare professional swabbing the back of a person’s nose or throat—although companies are developing kits that use saliva samples, which are easier and safer to collect than a swab. The sample is then mixed with a solution that breaks the virus open and frees specific viral proteins. The mix is added to a paper strip that contains an antibody tailored to bind to these proteins, if they’re present in the solution. A positive test result can be detected either as a fluorescent glow or as a dark band on the paper strip.
A typical antigen test starts with a healthcare professional swabbing the back of a person’s nose or throat—although companies are developing kits that use saliva samples, which are easier and safer to collect than a swab. The sample is then mixed with a solution that breaks the virus open and frees specific viral proteins. The mix is added to a paper strip that contains an antibody tailored to bind to these proteins, if they’re present in the solution. A positive test result can be detected either as a fluorescent glow or as a dark band on the paper strip.
Most antigen tests for COVID-19 detect the spike glycoprotein present on the surface of the SARS-CoV-2 virus. In rapid test format, viral antigen is directly identified by the immobilized coated SARS-CoV-2 antibody on the test device. The nasopharyngeal swab sample is collected in an extraction buffer available in the kit.
The interpretation of test results in rapid antigen test does not require specialized instruments and are usually presented within 30 minutes. Its counterpart, the RT-PCR COVID-19 test, though highly accurate, requires complex set-ups with bio-safety compliance and takes longer to deliver a diagnosis result.
This leads to a longer turnaround time while the number of cases rise. In contrast, the latest introduction, Feluda, the Tata CRISPR COVID-19 test, powered by CSIR-IGIB uses an indigenously developed, cutting-edge technology for detection of the genomic sequence of SARS-CoV-2 virus. Feluda has very recently received regulatory approvals from DCGI for commercial launch.
 As per ICMR guidelines, it meets high quality benchmarks with 96 percent sensitivity and 98 percent specificity for detecting coronavirus. According to the Ministry of Science & Technology, the Feluda test (short for FnCas9 Editor Linked Uniform Detection Assay) is the world’s first diagnostic test to deploy a specially adapted Cas9 protein to successfully detect the virus causing COVID-19.
As per ICMR guidelines, it meets high quality benchmarks with 96 percent sensitivity and 98 percent specificity for detecting coronavirus. According to the Ministry of Science & Technology, the Feluda test (short for FnCas9 Editor Linked Uniform Detection Assay) is the world’s first diagnostic test to deploy a specially adapted Cas9 protein to successfully detect the virus causing COVID-19.
Priced at Rs 500, it claims it can deliver a result in 45 minutes, give similar sensitivity and specificity as RT-PCR, requires a basic widely available PCR machine and does not require extensive trained manpower. Although the antigen test offers results in the least amount of time, there are certain limitations. The antigen tests do not amplify the protein signal and are therefore inherently less sensitive. However, the reports issued till date show that rapid antigen test may have relatively moderate sensitivity but provide high specificity. Despite its limitations, antigen tests enable early implementation of isolation protocols.
The value of rapid Ag tests has also been highlighted in WHO guidance 2020, especially in areas where there is a significant community transmission and/or nucleic acid amplification-based diagnostic (NAAT) testing is either unavailable or test results are considerably delayed.
Recognizing the need to test a greater number of samples and pick up the pace to Test-Track-Treat for early detection and containment of the COVID-19 outbreak, the healthcare industry is working unanimously with public health sector to scale up the testing infrastructure. Several small-cap companies have also begun developing and selling rapid Ag test devices. A large influx of products from smaller or recondite medical technology companies has flooded the market, creating a diverse range.
Antigen assays that may be used at home like pregnancy tests
Several experts have promoted the idea of developing an antigen test that is cheap and simple enough to use at home, without a health-care worker administering it.
What is needed is something as easy as a pregnancy test. A few companies are developing simple paper-strip antigen tests. But drug regulators have not yet approved them for emergency use.
Beyond concerns about costs and availability, researchers worry that, with an over-the-counter test, people who get positive results might not follow up with public-health authorities, so their contacts won’t be traced.
 Another risk would be people gaming the system, for example, getting someone else to take their test, so they can be sure of a negative result and avoid quarantine. Without incentives such as freely available tests and a living salary for those who have to isolate, testing and self-isolation could become a luxury reserved for wealthier people, others have argued.
Another risk would be people gaming the system, for example, getting someone else to take their test, so they can be sure of a negative result and avoid quarantine. Without incentives such as freely available tests and a living salary for those who have to isolate, testing and self-isolation could become a luxury reserved for wealthier people, others have argued.
Another concern is that people will get a false sense of security from tests that have only limited accuracy.
Indian market dynamics
In India, COVID tests comprise 50 percent RT-PCR tests, 40 percent antigen tests, and 10 percent TrueNAT and CB-NAAT, the latter two being TB tests with kits validated for COVID. However, over the last couple of months, rapid antigen tests become more popular and now account for 60 percent of all coronavirus tests in India. Such a high use could misrepresent the actual spread of the infection.
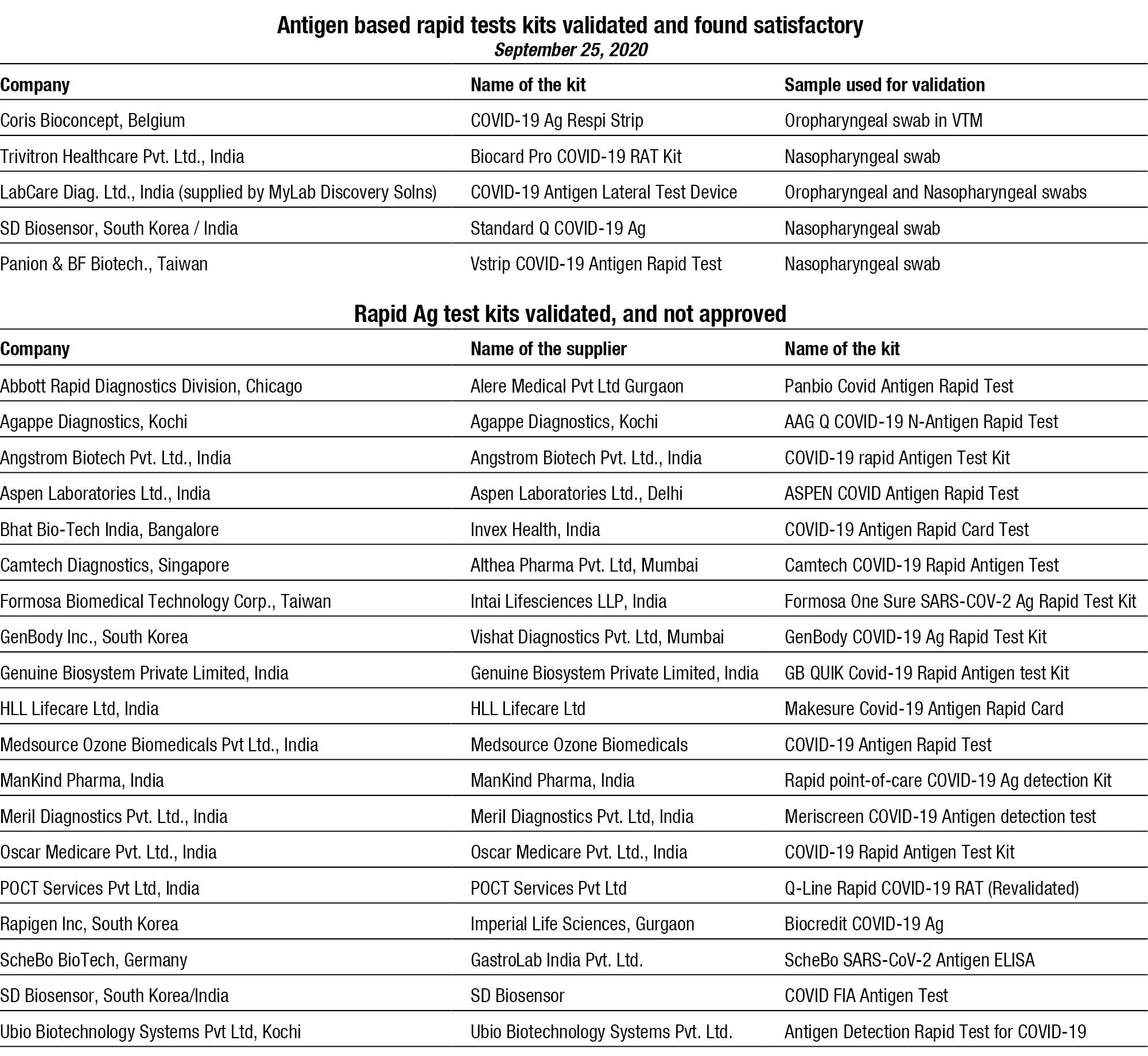
ICMR has so far validated 26 antigen-based rapid tests kits and 5 have been found to be satisfactory. The apex body has given permission that the US-FDA approved antigen-based rapid tests may be used directly, after due marketing approval by DGCI.
The minimum acceptance criteria for sensitivity and specificity of Rapid Ag test kits is more than 50 percent and 95 percent for the kits validated as a point-of-care Test (POCT) without transport to a laboratory setup.
For the kits validated in a laboratory setup with samples collected in Viral Transport Medium (VTM), the sensitivity should be 70 percent and above, while specificity has to be 99 percent and above. Sensitivity of the test is the extent to which true positives are detected and specificity indicates the tests should produce no false positives.
LabCare Diagnostics Ltd., India (supplied by Mylab Discovery Solutions) was the first to receive the commercial approval from ICMR for its Pathocatch COVID-19 antigen rapid testing kit.
Other test kits validated and being conducted in India are from SD Biosensor, an Indo-Korean company; Coris BioConcept, Belgium; Panion & BF Biotech, Taiwan; and Trivitron Healthcare Pvt. Ltd, India.
COVID-19 Ag Respi-Strip (Coris BioConcept) involves a different methodology of testing as compared to the other two antigen testing kits approved by ICMR till now. This test cannot be performed bedside and requires a BSL-2 set-up for running the test.
The rapid antigen test is expected to bridge the gap in current testing protocol by providing a faster and on-site diagnosis, especially in resource poor settings. Besides, when combined with more accurate RT-PCR, it aids in ramping up tests per day capacity in India. It cannot be used as a generalized test (despite the claims by many manufacturers) and sample has to be collection in a recognized setting.
In containment zones, people who are symptomatic with or asymptomatic but are direct and high-risk contacts of a confirmed COVID-19 case are eligible for this test.
In addition, a high-risk asymptomatic person with underlying comorbidities, or an individual seeking hospitalization for surgery/procedure can be tested in a hospital setting. Also, the healthcare individuals, or any person who is at high risk may benefit from this test.
ICMR’s advisory on COVID-19 testing strategy in India includes routine surveillance in containment zones and screening at points of entry with rapid antigen test (RAT) and RT-PCR or TrueNAT and CBNAAT, in order of priority.
All symptomatic people with influenza like illness (ILI), including healthcare workers and frontline workers, and asymptomatic individual who are direct and high-risk contacts (in family and workplace), including elderly ≥65 years of age, immunocompromised people, and those with co-morbidities, of a laboratory confirmed case need to be tested once between day 5 and day 10 of coming into contact.
Ideally all people living in a containment zone are tested by RAT, particularly in areas where there has been widespread transmission of COVID-19 infection.
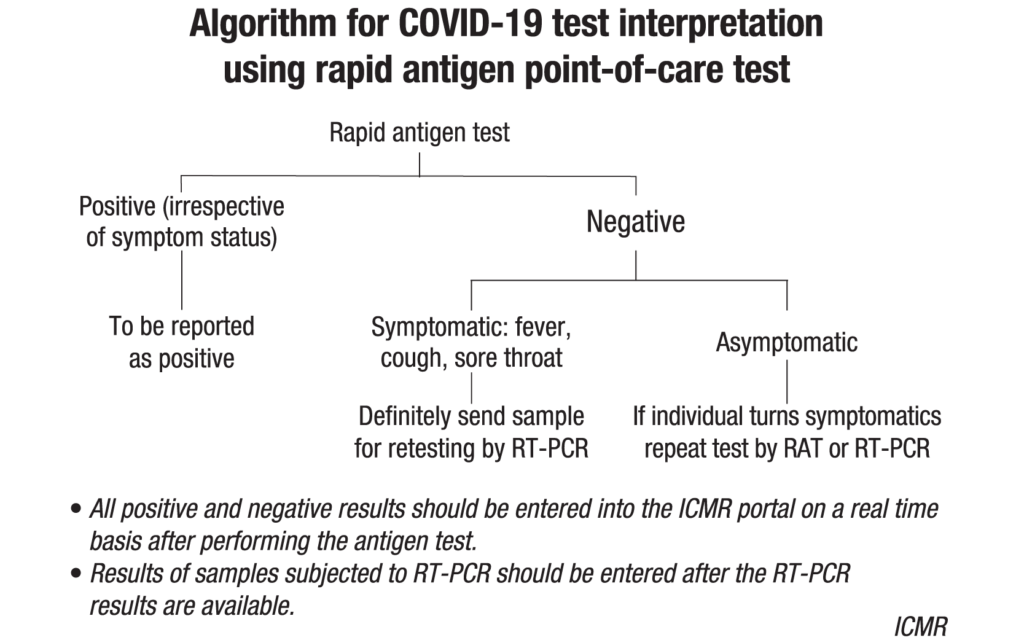
In hospital setting, rapid antigen test and RT-PCR are recommended for asymptomatic patients undergoing surgical/non-surgical procedures, and pregnant women in/near labour who are hospitalized for delivery. In all cases, if symptoms develop following a negative RAT test, a repeat RAT or RT-PCR is recommended.
To be the devil’s advocate, while the switch to rapid antigen testing by so many states in India may satisfy performance targets and meet public demand for more testing, they do run a real risk of not revealing the true extent of the outbreak, unless backed up by continued PCR testing!












