International Circuit
Singapore launches CLS for medical devices sandbox
The CLS(MD) was first announced in October last year during the Singapore International Cyber Week 2022. As reported in our previous alert, the objective of the scheme is to incentivize medical device manufacturers to adopt a security-by-design approach and enable consumers and healthcare providers to make more informed decisions about the use of such devices based on their cybersecurity provisions.
The CLS(MD) covers medical devices that handle personal identifiable information and clinical data, or that are able to connect to other devices, systems and services.
Launch of CLS(MD) Sandbox
Following an industry consultation earlier this year (as reported in our previous alert), the CLS(MD) sandbox was launched on 20 October 2023. It will run for a period of nine months.
During this period, medical device manufacturers will be able to apply to participate in the sandbox and have their medical devices put through different assessments to attain a rating under the CLS(MD).
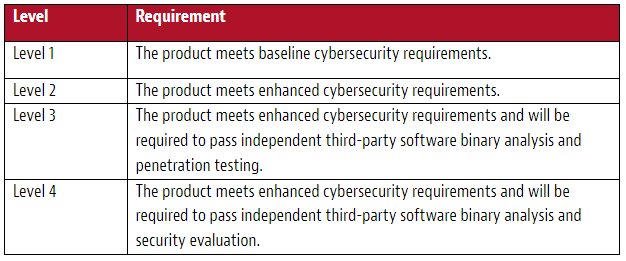
To recap, there are four levels of rating under the CLD(MD), with Level 4 being the highest rating. Each additional level represents an additional level of testing and assessment for the medical device. The refined requirements to be implemented in the sandbox are as follows:
The sandbox will allow manufacturers to test out and provide feedback on the requirements and application processes for the CLS(MD) ahead of its launch. Feedback and learning from the sandbox will be used to refine the requirements and operational workflow of the scheme where necessary. Global Compliance News












