Immunochemistry Instruments and Reagents
Taking sensitivity and flexibility to new levels in immunochemistry
From cutting-edge assay technologies to advanced automation platforms, the industry is evolving rapidly, with a focus on delivering faster, more accurate, and more flexible testing solutions.
The immunochemistry industry has been at the forefront of diagnostic and research technologies for several decades. Immunoassays are widely used in the detection and measurement of proteins, hormones, drugs, and other biomolecules in various sample types, including blood, urine, saliva, and tissue. They have numerous applications in clinical diagnostics, pharmaceutical research, biotechnology, food testing, and environmental monitoring.
Immunochemistry technology has undergone significant advancements over the years, and recent innovations have led to improved sensitivity, specificity, speed, and cost-effectiveness. These advancements have enabled the development of more accurate and efficient assays, making it possible to detect lower levels of analytes in smaller volumes of samples. Additionally, automated immunoassay platforms have made it easier to handle large sample volumes, reducing the turnaround time for analysis and improving laboratory efficiency.
The increasing prevalence of chronic diseases and the growing demand for rapid and accurate diagnostic tests have been driving growth. The use of immunoassays in point-of-care testing and home testing kits is also expected to increase, providing patients with convenient and affordable testing options.
Indian market dynamics
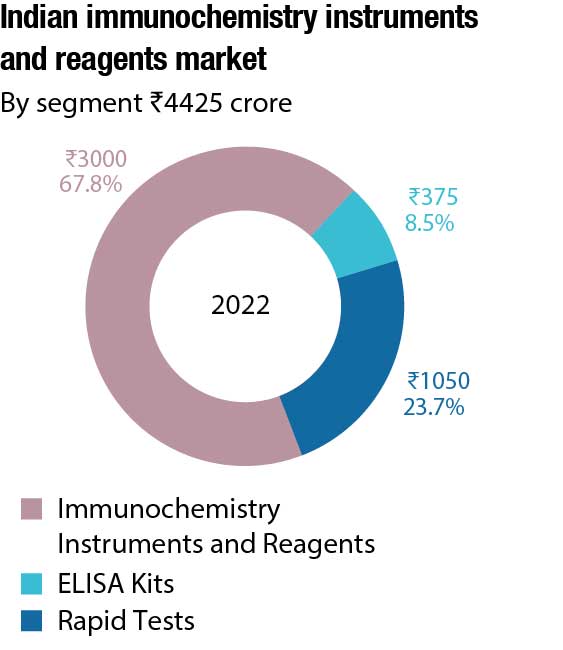
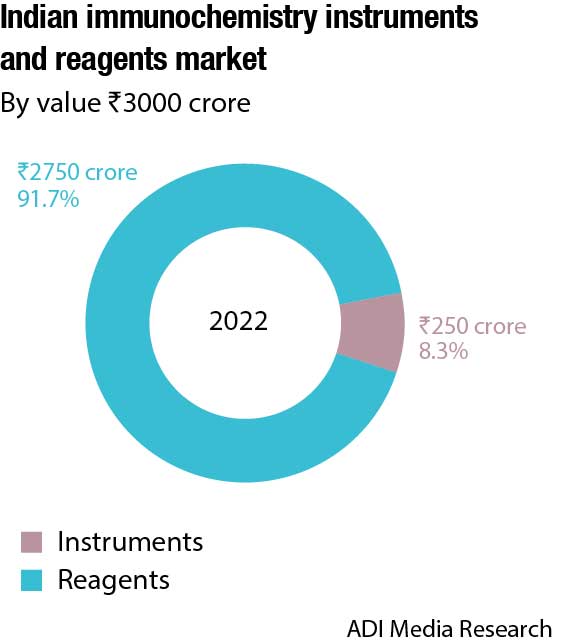
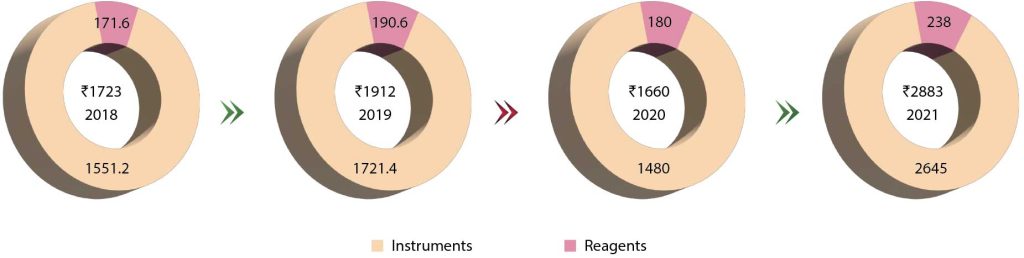
The Indian immunochemistry instruments and reagents market in 2022 is estimated at ₹4425 crore. Reagents continue to dominate the segment at ₹2750 crore, with instruments at ₹250 crore. The instruments and reagents are poised at a 7.5 percent CAGR. Additionally, the rapid tests’ market is estimated at ₹1050 crore and ELISA at ₹375 crore in 2022.
Roche leads the market by far. Abbott, Beckman Coulter, OCD, bioMérieux, and Siemens are also aggressive in this segment. Agappe is seeing success with its Maccura range and CPC with YHLO. Snibe and Mindray are also gaining popularity. The market continues to largely rely on rentals, with very few outright sales.
Transasia’s strength is in ELISA readers and washers. The company saw a 27 percent increase in revenue in the segment in 2022 over 2021. Orders from the government were received from the states of West Bengal (₹2.5 crore), North-East (₹1 crore), Rajasthan (₹0.24 crore), Gujarat (₹0.4 crore), KMSCL, Kerala (₹1.8 crore), and Tamil Nadu (₹1.3 crore). The company is working on developing its own ELISA instruments, targeted at tier 2 and 3 cities that will be able to read both on microplate and micro strip, and the strip can also be incised for just 4 tests to save cost. The launch is scheduled for November 2023.
| Indian Immunochemistry Instruments and Reagents Market–2022 | ||||
| Tier 1 | Tier 2 | Tier 3 | Tier 4 | Others |
| Roche | OCD, Siemens and Abbott |
Beckman Coulter and bioMérieux |
Snibe, Mindray, Agappe (Maccura), and Transasia* |
Tosho, CPC (YHLO), Autobio Diag, and Bio-Rad* |
| *Elisa
Vendors are placed in different tiers on the basis of their sales contribution to the overall revenues of the Indian immunochemistry market. |
||||
|
ADI Media Research |
||||
Some of the recent trends that are gaining acceptance are high-throughput automated equipment, multiplex assays, and customized reagents. Introduction of new biomarkers on the same platform is seeing intense competition amongst the prominent players. As the technology continues to evolve, the potential for automation to transform the field of immunochemistry in India is immense.
Global market
The global immunochemistry market size is estimated at USD 30.02 billion in 2022, and growing at a compound annual growth rate (CAGR) of 5.31 percent from 2023 to 2030 by Grand View Research.
Reagents and kits contributed to the largest market share of 66.1 percent in the immunochemistry market for 2022. The segment is expected to maintain its lead over the next 7 years. The large share can be attributed to high demand for immunoassay reagents and kits for diagnosis purposes with the growing prevalence of infectious and autoimmune diseases, alongwith the approval and launch of novel immunoassay kits.
The software and services segment is anticipated to register a CAGR of 5 percent. This can be attributed to the increased availability and high demand for cost-effective immunoassay services in the developing markets.
By technology, enzyme-linked immunosorbent assay (ELISA) segment held the largest market share of 63.93 percent in 2022, generating a revenue of USD 19,190 million. Repeated usage in the detection of infectious and chronic diseases, food allergies, and drugs of abuse among other applications, are major key drivers. Some of the major advantages of this method over immunoelectrophoresis and immunodiffusion are reduced assay time, quantitative results, and the requirement of limited antisera for analysis.
By application, the infectious diseases testing segment held the largest market share of 28.79 percent in 2022, and is anticipated to grow at the fastest rate in coming years. This high share can be attributed to the rise in the incidence of infectious diseases, such as HIV, malaria, influenza, and the novel Covid-19. The launch of new products in the field of infectious disease testing is further driving the market.
Based on end-use, hospitals segment dominated the overall market in terms of revenue in 2022 with 32.77 percent market share. The rise in the number of hospitals has led to the high growth of this segment. With the ongoing changes in the healthcare industry, the need for hospitals with advanced facilities has increased.
Clinical interpretation of diagnostic tests is dependent on assay analyzers and the presence of a technical and skilled workforce. Results of assays are affected by the time taken for interpretation. Thus, technological advancements have led to an increase in the number of automated instruments and equipment used in laboratories. Automation has benefits, such as higher efficiency and usage of less space and labor in laboratories. Thus, the market is witnessing lucrative growth as some market players are introducing automated laboratory systems and point-of-care compact, portable assay tests.
North America generated the highest revenue in immunoassay market with 39.45-percent share in 2022. This can be attributed to the rising demand for diagnostics due to the increase in the incidence of cancer and the easy availability of technologically advanced diagnostic techniques. In addition, the high incidence of infectious diseases in the region, such as tuberculosis, and influenza, is boosting the demand for detection and treatment.
Asia-Pacific region is projected to witness rapid growth with 6.81 percent CAGR as a result of increasing penetration of leading players in the region. In addition, the growing adoption of innovative laboratory techniques and procedures for faster investigation and diagnosis of infectious and chronic disorders is one of the factors driving the market in the region. Moreover, the introduction of molecular kits and the increase in demand for reagents used in diagnosis are key factors driving the market.
The overall market for immunochemistry is highly competitive and growing slowly, but has specific areas of opportunity that will be maximized. Many of the market’s tests are mature and have been available for years or decades. Significant growth opportunities in the immunoassay market may be possible through further innovations. This includes both new platforms and novel biomarkers. The attractive opportunities these innovations may make possible have attracted many diagnostics companies. Facets, such as economics and increased health insurance availability, are fueling market growth in developing countries, making them promising market segments. Many companies are targeting these markets, including major worldwide diagnostic companies as well as smaller ones. Future testing growth in these markets will occur in the existing technology (ELISA and lateral flow tests).
Recent advancements in immunochemistry diagnostics

Ketan Patel
Group Product Manager-Immunology,
Transasia Bio-Medicals Ltd.
Discoveries in immunochemistry have provided cost-effective diagnostic tools for faster and more accurate results.
The development of immunoassays began with the identification and quantification of various hormones, metabolites and infectious agents within different body fluids. Continuous developments in key areas of immunoassays have been observed, which include:
- Improvement in methods that deliver high intensity signals;
- Replacement of competitive formats to non-competitive format for smaller molecules;
- Low background interference; and
- Improvements in automation by introducing low throughput instruments to bring democracy in immunoassay.
Similarly, Point-of-Care Testing (POCT) with multiplexing capabilities has gained more popularity, as these tests are simple enough to operate at the primary care level and in remote settings where there is no laboratory infrastructure.
Technology trends
Chemiluminescent immunoassay (CLIA), being the most sensitive technique with a wide range of parameters and demanding the least human interventions, has gained reputation in the market. Introduction of direct CLIA technique that utilizes direct luminophore markers like acridium and ruthenium esters has added significant value to the sensitivity of the assays.
Fluorescent immunoassays (FIA) is another technique that is emerging in immunochemistry. A number of breakthroughs have been made in recent years that have made FIA-based platforms available for patient care as point of care. This fluorescence detection when incorporated with lateral flow system along with a low cost analyser that reads and quantify the results, the assay performance is further increased.
ELISA is losing its market share to CLIA and FIA, as most blood banks are updating from conventional ELISA to CLIA for TTI testing.
Technological advancements in immunochemistry such as super paramagnetic immunoassays, Ultra-sensitive immunoassays, multiplexed immunoassays have increased dependency on this segment. Combining biochemistry or flow cytometry with immunoassay offers cutting-edge technology to modern state-of-art laboratories that churn huge sample loads.
Indian immunochemistry market is growing by highest CAGR amongst all other segment which is 7.5 percent with total market share of ~ Rs.3100 crore in 2022-23. Amplification of the immunochemistry market solely depends on development and introduction of new biomarkers on the same platform. The market is expected to witness a stiff competition amongst the prominent players.
The prominent manufacturers operating in the market are Abbott Laboratories, Beckman Coulter, Mindray, Ortho Clinical Diagnostics, bioMérieux, Thermo Fisher Scientific, DiaSorin S.p.A., Bio-Rad Laboratories Inc., Alere Inc., Sysmex Corporation, Quidel Corporation, Roche Diagnostics Limited, Siemens Healthcare, and Becton, Dickinson & Company. Various strategies, such as expansions, collaborations, partnerships, mergers and acquisitions, joint ventures, and others have been adopted by prominent manufacturers to gain a strong foothold in the market.
Latest trends

Thomas John
Managing Director,
Agappe Diagnostics Ltd.
Nephelometry and turbidometry are two commonly used techniques in immunochemistry for the measurement of antigen-antibody interactions, which measures the amount of light scattered by antigen-antibody complexes in solution.
Technological advanced highly efficient instruments and relevant disease specific tests help the medical fraternity for the early and accurate diagnosis of patients to make effective treatment. Immunochemistry analysers have proved to be very effective tools to diagnose the diabetes, inflammation, fertility issues, tumour, bone metabolism disorders and the other kinds of infectious and chronic diseases.
POCT devices that utilize nephelometry and turbidometry techniques are being used for the rapid and accurate diagnosis of various diseases, in conjunction with other technologies, like microfluidics and mass spectrometry, to increase sensitivity and specificity. These assays have become increasingly preferred over last two decades, enabling highest sensitivity and specificity.
High-throughput automated equipment is becoming increasingly popular in India, which can process large numbers of samples quickly and accurately and ideal for larger clinical laboratories.
Multiplex assays have gained popularity in recent years, helping simultaneous measurement of multiple analytes in a single sample, making them more efficient and cost-effective.
Automation, miniaturisation, multimodal detection in nephelometry and turbidometry assays have led to increased accuracy and reproducibility. Automated systems also allow for high-throughput analysis, which is essential in clinical laboratories that process large numbers of samples.
Customized reagents are becoming increasingly popular in India as researchers and clinicians which can improve the accuracy and specificity of immunoassays.
Growing emphasis is being laid on the validation and standardization of nephelometry reagents and equipment, using reference materials and protocols ensure consistency and accuracy.
Agappe is striven to provide the advanced techniques in the immunochemistry instrument and reagents with the advanced Nephelometry system like Mispa i2 and Mispa i3, offering more than 25 ready to use LEIT reagents that can also perform with any automated or semiautomated biochemistry analysers including key tests like Vitamin D and special parameters like Cystatin C, KPPA, LAMDA light, Immunoglobulins IgA, IgE, IgG, IgM; Lp(a), APO -A, APO-B, C3, C4, Ceruloplasmin, D-Dimer Ferritin etc. Agappe offers very cost-effective, convenient pack sizes that can suit low throughput labs as well as higher turnover labs, with pre-calibrated reagents.
Recent advancements
From the basic in-vitro study of a specific biomolecule to the diagnosis or prognosis of a specific disease, one of the most widely used technology is immunoassay. By using a specific antibody to recognize the biomolecule of interest, relatively high specificity can be achieved by immunoassays such that complex biofluids can be analyzed directly. In addition to the binding specificity, the other key features of immunoassays include relatively high sensitivity for the detection of antibody-antigen complexes, and a wide dynamic range for quantitation. Over the past decade, the development and applications of immunoassays have continued to grow exponentially. Some of the latest technologies for the development of new immunoassays include:
Nanozymes. Immunoassay is a highly selective diagnostic method, established by the specific immune reaction between antigens and antibodies. There are many kinds of immunoassays with various detection signals, such as colorimetric immunoassays, fluorescence immunoassay, chemiluminescent immunoassay, electrochemical immunoassay, electrochemiluminescence immunoassay, and so on. Because of the merits of easy operation, high sensitivity and selectivity, automated high throughput, and visual inspection, ELISA has become an attractive method in immunoassay, which has widespread applications, including clinical diagnosis, food safety, and environmental monitoring, whereas the natural enzymes labels usually suffer from some intrinsic drawbacks like low stability, high cost, and difficult storage. Thus, the seeking of enzyme substitute in immunoassay is of vital significance.
With the development of nanotechnology and biotechnology, a new type of nanomaterial, with intrinsic enzyme-like characteristics as nanozymes, has been exploited in recent years. Nanozymes not only show high catalytic activity with tunability but also eliminate the defect of natural enzymes, exhibiting the merits of easy preparation, low cost, and high stability. Thus, nanozymes have become a charming substitute for natural enzymes. By replacing natural enzymes with nanozymes, modified immunoassays based on nanozymes have emerged, which exhibit higher sensitivity and stability than traditional enzyme-immunoassays. So far, although a series of reviews have been published for the summarization of immunoassays with various detection signals, only a few focus on the employment of nanozymes. To attract the researchers interested in this charming field, a comprehensive summary and analysis of the advances of immunoassays based on nanozymes is urgently needed.
It is time to move the traditional nanozyme-based immunoassays out of the lab. By integrating micro devices, such as smart phones, micro-fluidic chips with nanozyme-based immunoassays, point-of-care testing, and on-site analysis can be realized, which can improve the detection efficiency, simplify the operation procedure, and reduce the cost. Overall, the nanozyme-based immunoassays are becoming a promising area of research and have potential prospects in a wide range of applications.
Multiplex assays. For years now, scientists have adopted multiplex assays to generate more robust and reproducible results compared to separate, single-plex immunoassays like ELISAs. There are a few different approaches to multiplexing, but in general, multiplex assays improve the quality of results by testing many targets or analytes in a single reaction. Additionally, using identical experimental conditions for all of the analytes the test makes the results generated between analytes more easily comparable. Depending on the multiplex approach used, other advantages of these higher-throughput systems often include miniaturized reactions that lower sample input requirements and reduce reagent use. They also minimize hands-on time, allowing scientists to move on to other tasks, and reduce the cost of each experiment. Importantly, they may detect hundreds of analytes in a single run.
Recently, a new feature of multiplexing technology has been developed to reduce variability in immunoassays further. The dual-reporter feature allows scientists to collect two different measurements from each analyte in a sample. This would be particularly helpful when the measurements come from related elements, such as detecting antibody isotypes, the presence or absence of post-translational modifications in nucleic acids or proteins, or free versus bound drug in a sample. In a recent study, a multiplex approach was used to detect both IgG and IgM antibodies to three antigens of the SARS-CoV-2 virus. The technique involved a bead-based multiplexing system, in which the dual-reporter channels captured two distinct signals from each individual bead, effectively doubling the data produced from the study and ensuring that experimental conditions for both antibody isotypes were identical. Data generated with this method provided a more accurate and complete view of the immune response to the virus, compared to traditional methods.
Advance on the new generation of small molecule detection technology

Prof. Praveen Sharma
Scientific Consultant,
Snibe Co., Limited
Chemiluminescence immunoassay (CLIA) is an assay that combines the chemiluminescence technique with immunochemical reactions. In recent years, CLIA has gained increasing attention in different fields, including life science, and clinical diagnosis for its high sensitivity, good specificity, and wide linear range. The CLIA can be used for detection and quantification of antibodies and, to measure the concentration of antigens in immunoassays.
Sandwich and competitive methods are the two main types used in small molecule diagnostics. For most of the small molecules, due to their compact structure, their molecular mass and steric hindrance are limited. Small molecule analytes usually have only one epitope and no immunogenicity, so it is difficult to produce an immune response and therefore, only competition methods are used. The principle of the competition method is that the antigen in the specimen competes with a certain number of labeled antigens and binds to solid-phase antibodies. However, the affinity between the antigenic analog and the natural small molecule antigen for the same antibody strain is often difficult to keep the same, which also leads to the wrong results.
Recently, a new generation of small molecule detection technology that gained a lot of attention is using the sandwich method instead of the traditional competitive method to complete the detection of small molecules. Differing from the usual sandwich method, which combines two antibodies to one antigen, the small molecule sandwich method is achieved by developing antibodies against the immune complex which consists of target antigens and primary antibodies. A special antibody will not recognize either the target antigen or the coated antibody individually, only the complexes formed by them. Compared with the competitive method, the sandwich method has higher sensitivity, precision, and accuracy. Even in low-concentration samples, this technology also achieves precise tests.
Nowadays, manufacturers are already using this technology for 25-OH vitamin D testing and achieving good results consistent with the reference measurement procedure (LC-MS). Relevant studies have shown that this breakthrough sandwich method can be used to measure vitamin D with better accuracy than the competition method. In the future, with the advancement of technology, it is believed that this technology will be widely applied for the detection of smaller molecule analytes.
Artificial intelligence (AI) and immunoassays. Rapid changes in diagnostic sector, coupled with parallel advances in digital transformation technology in clinical chemistry and immune assay platforms, have stimulated the evolution of approaches for AI and robotic elements in routine laboratory process flow. Laboratory processes need to be streamlined to ensure provision of reliable and timely test results, appropriate alliance with brain-to-brain loop, thus enhancing quality of care and patient safety.
Thus, implementation of digital transformation in the immunology laboratory improves revenues, suggests patient specific next steps, tests utilization, improves quality, standardizes treatment protocols as per local and international guidelines, improves patient satisfaction, provides patient-specific interpretation and next steps, improves standardized care by flagging patients, applies risk algorithms, and provides better interpretation.
Electrochemical lateral flow immunoassays. The discovery of electroactive nano-biomaterials and the development of flexible electrodes have increased the interest in applications of integrated electrochemical lateral flow immunoassays (eLFIAs), which integrate electrochemical nanotags and flexible electrodes on test strips that can easily detect small biomolecules. Compared with colorimetric, optical, magnetic, and other highly sensitive detection methods, the electrochemical detection technique is well developed with high sensitivity, selectivity, and repeatability. Moreover, the increasing compatibility of interfaces with miniature potentiometers has allowed electrochemical sensors to become more integrated, automated, and intelligent, highlighting their huge potential in future developments.
No-wash ELISAs. While immunoassay techniques continue to improve, there is a growing need for more advanced capabilities that can deliver faster throughput, greater sensitivity, and better ease of use. Some of the latest developments include no-wash ELISAs with reduced-step protocols, along with new automated platforms designed to eliminate user error and accelerate time-to-results. Meanwhile, the ability to miniaturize immunoassays down the nanoliter level is enabling researchers to generate more data from smaller amounts of samples and reagents.
Immunoassay affordability is an imperative component to expanding access to healthcare

Dharampal Sharma
National Product Manager – CLIA,
Mindray Medical India Private Limited.
The automation of immunoassays is not a new trend, it has already witnessed decades of innovation in diagnostic laboratories. Improved performance, greater sensitivity, and better uniformity reduce reagent consumption requirements or cost per sample. Enhanced speed and reduced assay time are the key reasons for the increasing demand. Sometimes lack of access to quality diagnostics remains a major contributor to health burden in resource-limited settings. Currently, highly accurate tests require complex instrumentation and are not easy to perform outside of sophisticated laboratories in urban settings where trained technologists are available. These tests are neither accessible nor affordable to patients at the lower levels of the healthcare system. There can be no better example than India to illustrate the need for medical technology for improving healthcare delivery. In the second most populous country of the world, the supply of healthcare services falls significantly short of the demand. Existing healthcare delivery mechanisms are inadequate to meet the ever-growing needs of the Indian population, especially in smaller towns/rural areas.
Mindray is committed to a Healthier Bharat by making healthcare affordable and accessible through cutting-edge technology and innovations. To make immunoassay more affordable Mindray acquired Hy Test, a global leading provider of antibodies and antigens.
In an increasingly competitive environment, the challenges facing both large laboratories and core laboratories are increasing daily. Despite overwhelming workloads, they need to deliver accurate results fast, improve productivity, and bring down costs, while also enhancing their business performance.
Mindray CLIA Solution. Mindray offers holistic solutions in immunoassays that cater to all types of customer’s needs. Instruments ranging from CL-900i (having a throughput of 180 T/H) to CL-6000i series (having a throughput of 480 T/H) are available.
Mindray has a complete profile of CLIA solutions for thyroid, reproductive, metabolic, tumour, iabetic, and anemia markers alongwith many more clinically significant markers. We will be soon launching new immunoassays markers for Sepsis, TORCH and many more. To serve the high workload sites we can upgrade CL-2000i & CL-6000i, high end automated CLIA systems, and integrate them with the biochemistry platforms to make SAL 6000 & SAL 9000 modular workstations.
Miniaturization and automation not only improve the efficiency of critical steps along the discovery continuum, but also help remove many potential sources of variability to provide more accurate and reproducible results. While different labs require different degrees of automation, the underlying need for greater precision, throughput, and reproducibility remains a key driver.
Digital ELISA. It allows researchers to dig deeper with higher sensitivity and less sample. Compared with traditional ELISA, digital ELISA takes advantage of much, much smaller wells, those on the order of 15 femtoliters in volume. In this environment, a single molecule can produce a signal strong enough to be detected, if the background noise is minimized. Additionally, if each well contains at most one molecule, and you can count up the number of wells that are giving a signal, then you have an extremely accurate measurement of the total number of target molecules in your sample.
Another significant advantage of this approach is its flexibility in terms plex and sample type. It is capable of either single-plex or multiplex detection, and because it can detect proteins at ultra-low concentrations, researchers have their choice of sample type, be it blood, urine, serum, or cerebrospinal fluid.
The increased sensitivity and minimal sample volume allow for the detection of proteins that were difficult or impossible to measure in older types of immunoassays. The ability to customize and automate digital ELISAs offers a considerable advantage over traditional ELISAs and western blots because it reduces time and cost while quickly delivering a wealth of information.
Sensitivity, reproducibility, and data output. One of the main goals in the development of immunoassay technology is to improve its sensitivity, which refers to the ability to detect lower concentrations of the target molecule. This is important because some molecules of interest may be present at very low levels, especially in clinical samples, and detecting them accurately can be challenging. As such, advances in technology have led to the development of more sensitive assays that can detect lower concentrations of target molecules, improving the accuracy of diagnosis and research outcomes.
Immunochemistry – Need of the day

Dr Kanchan Sonone
Consultant, Biochemistry and Laboratory Director,
Wockhardt Hospital, Nashik
In medical science, there have been many advances, and one of the key factors in advancement is the immunochemistry technology, which has developed to detect levels of molecules, such as proteins, hormones, drugs, and other substances that control human body on the molecular level. Immunochemistry is an important tool for scientific research and a complementary technique for the elucidation of differential diagnosis, which are not determinable by conventional analysis. Immunochemistry witnesses extraordinary advancement in every aspect of basic and clinical research and this is due to technological advances, which enable quantitatively and comprehensively measuring immune response to genetic or environmental perturbations. Immunochemistry analyzers are becoming more and more popular these days as they have proved to be very effective tools to diagnose cancer, hepatitis, illegal drugs, fertility problems, infectious diseases, and many chronic diseases. Now-a-days, chemiluminescence immunoassay (CLIA) systems are gaining momentum, which in turn results in a decline in enzyme-linked immunosorbent assay (ELISA) tests. Due to stringent checks, rapid tests are also seeing a decline and gradually shifting to chemiluminescence.
Automated CLIA analyzers are now preferred by even standalone laboratories. Besides faster TAT (turnaround time), it offers other benefits, such as precision, ease of processing a large number of samples, and minimal manual intervention, leading to a streamlined workflow. Immunochemistry plays an important role in development of biomarkers too as growth in incidence and prevalence of infectious diseases, cancer, gastrointestinal and cardiovascular diseases, endocrine diseases, and many other chronic diseases is on the hike. The evolution of diagnostic and prognostic markers is significantly changing the clinical practice, and this can be demonstrated by the use of immunochemistry. The demand for immunochemistry is increased due to overall increase in healthcare expenditure, decreasing mortality, increased awareness, and lifestyle-related diseases.
Immunochemistry is the backbone of diagnosis and prognosis of diseases in healthcare system, and is thus the need of today for accurate and precise measurement of the sensitive parameters and for their rapid diagnosis.
Another important aspect of immunoassay technology is reproducibility, which refers to the ability of an assay to give consistent results when performed multiple times under the same conditions. This is important to ensure that the results obtained from an assay are reliable and can be used to inform decision making in clinical and research settings. Improvements in technology have allowed for more precise and consistent results to be obtained, reducing variability and improving confidence in the data generated.
Data output is another important aspect of immunoassay technology, as it refers to the amount and quality of information that can be obtained from an assay. The creation of increasingly complex and automated systems that can swiftly and accurately create enormous amounts of data is the result of technological advancements. This is especially beneficial in high-throughput applications where a lot of samples need to be examined quickly.
Finally, reducing sample volumes is an important goal in immunoassay technology. This is because some samples, such as those obtained from patients, may be limited in volume, and minimizing the amount of sample needed for analysis can be beneficial. Advances in technology have led to the development of microscale assays that require smaller amounts of sample while maintaining high sensitivity and reproducibility, allowing for more efficient and cost-effective analysis.
Outlook
The immunochemistry industry is poised for continued growth in the coming years, driven by a combination of technological advances and increasing demand for diagnostic and research tools.
One key area of growth is in the development of more sensitive and specific assays, which can detect lower concentrations of target molecules and provide more accurate results. This is particularly important in the field of clinical diagnostics, where accurate and timely diagnosis can be critical for patient outcomes.
Scope & trends in immunochemistry
![]()
Dinesh Wagh
Assistant General Manager-Automation,
Vector Biotek Pvt. Ltd.
(A Beacon Group Company)
Immunochemistry refers to the study of the chemical aspects of the immune system with the application of antigens or antibodies as chemical reagents. Immunochemistry is useful to treat various diseases such as heart disease, infectious disease, cancer, endocrinology, drug abuse testing, and drug development.
In the healthcare settings it is critical to diagnose, monitor and manage patient health effectively. Immunochemistry is proving it’s worth in this direction since last two decades, with development of reagents and instruments that provides qualitative, semi quantitative, and quantitative analysis of targeted analytes.
The global market for immunoassay is expected to reach at USD 49.2 billion by 2027, with CAGR of 4.3 percent during period of 2022 to 2027. Together with increase in demand due to rising incidences of illness, advancements in systems & technologies, demand for early-stage diagnosis of disease, growing awareness in the area of biotechnology & biopharmaceuticals are also the major contributor for tremendous market growth.
Beginning from the early days, RIA systems, right up to today’s most ultramodern systems developed in immunoassay have made this product line a major contributor amongst IVD products. Diverse immunoassay formats, analytical platforms, and systems along with complementary technologies have made ultramodern conversion possible. On the other hand, by incorporating advance technologies like microfluidics, it has further made the technology more user friendly, time saving and cost efficient. Use of Lateral Flow Immunoassay with microfluidics & electrochemical immunoassay are the most recent examples that shows the promising upward trends of business in this sector.
POCT which is the most inevitable methodology for providing better patient outcome at faster speed is one of the important areas. Detecting the presence of specific analytes by using LFIA techniques together with fluorescence measurement is becoming increasingly popular in diagnosis.
Wide variety of complex analytes are investigated for diagnosis, prognosis and treatment management using POCT Platforms. In this segment Beacon Group has its most active presence by providing IF based Quantitative POCT Immunoassay system which is showing the promising results for medical professionals to provide better and faster patient care for better healthcare outcome.
Another area of growth is in the use of immunoassay technology in research and development, particularly in the pharmaceutical and biotechnology industries. As new therapies and treatments are developed, there is an increasing need for accurate and reliable methods of measuring the effects of these treatments on biological systems. Immunoassays provide a powerful tool for this purpose, allowing researchers to measure changes in specific biomolecules in response to treatment.
In addition, the increasing availability of automated and high-throughput immunoassay platforms is expected to drive growth in the industry, as these platforms can process large numbers of samples quickly and efficiently, reducing the time and cost of analysis.
Despite these promising trends, the immunochemistry industry faces a number of challenges. The most significant is the increasing competition from alternative technologies, such as molecular diagnostics and next-generation sequencing. These technologies offer the potential for even greater sensitivity and specificity than traditional immunoassays, and may eventually replace them in some applications.
Technological advancements and emerging trends

KCS Negi
National Sales Manager,
Sowar Private Limited (Medical Division)
With continuous innovation and breakthroughs, testing has taken a new leap with latest state-of-the-art technologies available at affordable prices. And the emerging trends in immunochemistry industry have travelled a long distance with technological advancements, upgradation ruling it.
Immunochemistry analyzers are instruments that automatically run tests on samples from patients to detect any number of biologically an immunochemistry analyzer can be used by medical laboratories for a variety of tests, such as cancer, hepatitis, illegal drugs, fertility problems, sodium levels, endocrine function, and the detection of blood clots.
Major players in the immunochemistry diagnostic devices and equipment market are Abbott Diagnostics, Roche Diagnostics, Siemens Healthcare Diagnostics, Diamond Diagnostics, Dynex Technologies, Grifols, Hycor Biomedical, Immunodiagnostic Systems, Inova Diagnostics, and LabCorp.
The immunochemistry diagnostic devices and equipment market consists of sales of chemiluminescence immunoassay (CLIA) analyzers, immunofluorescence (IFA) analyzers, enzyme immunoassay (EIA) analyzers, radioimmunoassay (RIA) analyzers, enzyme-linked fluorescent assay (ELFA) systems, and multiplexed assay systems that are used in immunochemistry diagnostics.
The consumables are antibodies, antigens, enzymes, reagents, stains, buffers, disposables, and others. The various applications are endocrinology, oncology, cardiology, therapeutic drug development and monitoring, infectious disease testing, drugs of abuse testing, and others that are used by various end-users are hospitals and clinics, diagnostic laboratories, research labs, and institutes, biopharmaceutical and biotechnology companies, and others.
In addition, the development of various integrated clinical chemistry systems has immensely improved the efficiency of the analytical phase of clinical chemistry laboratory testing and led to further automation. For example, Getein 1100 is an Immunofluorescence Quantitative Analyzer from Getein Biotech Inc. for processing and analysis of Getein test kits including biomarkers for cardiovascular, inflammation, diabetes, thyroid function, fertility, bone metabolism, tumor, infectious disease, etc. Getein 1100 is used to measure the concentration of biomarkers in human whole blood, serum, plasma, urine, nasal swab, or saliva samples. The results can be used as an aid in clinical diagnostics of laboratory and point-of-care testing. It is applicable in emergency, critical care (ICU, CCU), outpatient, cardiology, inpatient wards, and clinical laboratories.
Increasing regulatory scrutiny of immunoassay technology, particularly in the field of clinical diagnostics also poses a challenge. As the importance of accurate and reliable diagnostic tests continues to grow, regulators are placing increasing emphasis on ensuring that these tests meet high standards of performance and quality.
Overall, the outlook for the immunochemistry industry is positive, with continued growth expected in the coming years. However, the industry will need to remain vigilant in the face of new challenges and changing market conditions in order to maintain its competitive edge.
The way forward in the next decade

Dr Nita Munshi
Ex-Director Laboratory,
Ruby Hall Group of Hospitals, Pune & NABL Assessor
In the present era, we have seen a meteoric rise in the number of immunochemistry tests requested for by the clinicians. Especially when we are living in a world of evidence-based medicine, where no disease condition is diagnosed without laboratory tests, the role of all lab tests is very important.
Till recent times, immunochemistry was an integral part of biochemistry. Gradually, the number of tests started rising, and hence the segregation.
Immunochemistry instruments have evolved with time. Initially, we used RIA for thyroid tests. Then, came the era of ELISA manual method – which required training on use of ELISA, pipetting precision, plotting graphs, and reading results, but was very cumbersome.
Then came immunofluorescence technology, which required special microscopes, training on preparing the assay and interpreting, and was cumbersome and prone to errors.
Immunoassay was the next improvement wherein semi-automated and automated equipment incorporated the reagents for various immunochemistry assays. The menu
expanded and so did the research for various tests and equipment. There are several companies now making instruments for immunochemistry.
Immunoprecipitation is another technique used for immunochemistry analytes.
What does the future hold? What does a lab professional expect from immunochemistry? What do clinicians expect from immunochemistry assays? What does the industry expect in return? These are some of the questions, which come to mind when we think of the way forward.
Immunochemistry tests have a huge menu. Labs expect that the equipment should cater to a wide range of tests. The process should be one of random selection of tests. There should be an option of loading emergency tests. The TAT should be as short as can be. If possible, the longest TAT and the shortest TAT should be very close.
All results should be precise and accurate.
Quality and integrity of samples should be maintained and results consistent, with refrigeration on board so reagents can be kept loaded. Footprint of the equipment must be as small as possible and easily movable, so not very heavy. There should be a warning, which is audible and readable, if there is a problem with QC or sample result. The reagents loading capacity must be enough to hold maximum number of reagents for the entire test menu, so there is no need to shuffle reagents. The reagent capacity should be adequate for at least 100–150 tests. Reagent inventory, LJ charts are already provided by most of the new equipment. But automatic reporting of results with a sophisticated software, cloud-based reporting, automatic email facility, should be available from the equipment. Ease of HIS and LIS integration is important. Use of water must be minimum with on-board water treatment and storage facility. And most important is consistency of results!
Clinicians’ expectations are minimal, huge test menu; short TAT; consistent and quality reports; and preferably reports available on WhatsApp or SMS. Most important of all is cost effectiveness of the test.
The industry expects that labs trust their product, for without a market share their production of equipment would be useless. They provide 24×7 service. Volume of sample taken must be minimum, especially for pediatric patients and patients with thin veins. They train their staff regularly on various aspects of the equipment. And most important, they minimize cost of production so that CPRR for each parameter is affordable for the lab, and subsequently the patient.
Ideal futuristic way forward would be centrifugation of sample on board; loading the sample which can perform both biochemistry and immunochemistry tests for the entire range of test menu, with minimum sample volume, shortest TAT; and release results after correlating with QC checks. And being able to store the samples on board after recapping them refrigerated. The software should make archival of samples easy in case more tests need to be added.
This will enable all the users – the lab, the clinicians, the management, and the industry to be on a win-win path!












