MB Stories
Understanding the epidemiology of infectious diseases

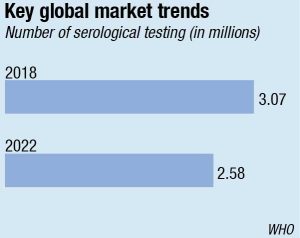
The Indian serological testing market that had steadily been seeing an increase every year saw a sharp dip of 15.2 percent in 2021. It is back on track now.
Antibodies are important clinical, immunological, and epidemiological biomarkers. They are stable circulating molecules, whose production requires multiple layers of cellular cooperation and genetic diversification with antigen-driven selection, making them highly specific reporters of both the inciting agent and the host’s immune capacity. At the same time, the enormous diversity of the antibody repertoire–and its possible antigenic targets–poses a challenge–traditional assays are able to quantify only a single antibody specificity per unit volume of material, and thus are unable to provide a comprehensive view of the response from a typical sample. Instead, the next generation of immune monitoring requires highly multiplexed but cost-effective approaches, capable of resolving antibody reactivity across large numbers of targets simultaneously by using a small sample volume.
Traditional serological assays, such as ELISA use immobilized antigen (e.g., peptides, proteins, and whole pathogens) and enzymatic or fluorescent detection to quantify antibody reactivity against a single target at a time. Although they can be highly quantitative, these assays are limited in their ability to provide breadth and resolution, because each additional target of interest requires the consumption of additional serum or plasma, which is often limiting, as well as other associated reagents, which can quickly inflate cost. To obtain high sensitivity, these assays also commonly use large, complex antigens with the potential for cross-reactivity among closely related targets. The use of encoded beads has expanded the multiplexity of serological assays to the range of 5–500 simultaneous antigens, but this remains incommensurate with the diversity of natural antibody responses. In addition, high-density peptide array technologies have been developed that allow hundreds to hundreds of thousands of peptides to be assayed simultaneously; however, these approaches have not yet achieved widespread uptake, probably in part because the need to manufacture and/or process one array for each sample can be cost prohibitive for larger-scale experiments.
In contrast, highly multiplexed serology can be achieved cost-effectively by solution-phase assays, in which antibodies are probed with highly diverse libraries of DNA-associated antigens, and binding is then quantified by measuring the relative abundance of each peptide by using high-throughput sequencing. Populations of phage particles, displaying defined libraries of peptides on their surface, represent a successful embodiment of this approach, and have been used to profile reactivity across the human proteome as well as the proteomes of all human-infecting viruses.
One of the big questions on the medical side is which antibodies provide protection against infection. This is where humanity found itself in 2020 – not knowing which were the right antibodies that the body needed to develop to respond to Covid-19. That information was needed to create an effective vaccine and, in the future, could help develop vaccination approaches that would broadly protect against coronaviruses like SARS-CoV-2, including those that we have yet to identify.
PepSeq. Jason Ladner, an assistant professor in the Department of Biological Sciences and the Pathogen & Microbiome Institute, was a leading innovator on PepSeq, a technology that allows scientists to test antibody binding against hundreds of thousands of protein targets at one time, instead of testing one at a time.
PepSeq predicated on the same general principle as phage display but involved a much simpler molecular construct–a covalent adduct of peptide and cDNA. A derivative of mRNA display, this process uses bulk enzymatic transcription and translation, followed by puromycin-mediated intramolecular coupling between the peptide and its encoding nucleic acid sequence to build high-complexity libraries representing antigen sets of interest that can be used to deeply profile polyclonal antibody repertoires. Advantages of this approach include the reduced potential for non-specific background binding, as well as the ability to construct libraries for highly multiplexed serology assays entirely in vitro.
A fully in vitro platform for highly multiplexed serology, using customizable DNA-barcoded peptide libraries, this novel approach details the team’s approach for designing and synthesizing custom PepSeq libraries, as well as how to use these libraries to conduct assays and interpret the data. The hope, Ladner said, is to provide a roadmap for scientists everywhere to use the protocol in their own research, moving us closer to answers on some major infectious diseases.
It is an important step forward as concerns about bioterrorism, zoonotic diseases, and the next pandemic are never far away and understanding these pathogens will help enable scientists to develop vaccines, as well as tracking their movement and evolution.
“Serology assays are important for diagnosing many human and animal infections,” Ladner said. However, traditional assays often lack sensitivity and/or specificity. Our approach can help to identify which proteins most commonly stimulate an antibody response during infection, and which epitopes are specific for the pathogen of interest rather than being cross-reactive across related pathogens.”
Ladner’s team is leading the development of bioinformatics protocols and custom software packages that will create the PepSeq libraries and allow for interpretation of data collected through the protocol. From a big-picture standpoint, having these kinds of conversations helps the field as a whole progress, Ladner said. It helps develop trust in the process and the results.
“Even if no one outside of PMI and TGen end up using PepSeq, we want the broader scientific community to trust that the results are reliable, and the best way to do that is through transparency about the process,” he said. “In this type of protocol manuscript, we can go into much greater detail about the methods than we can in a manuscript focused on the results of a particular research project that utilizes PepSeq.”
Serological testing has proven invaluable in diagnosing viral infections, autoimmune disorders, and certain cancers. Advancements in serological techniques and technologies have revolutionized medical diagnosis by providing increased reliability, accuracy, and efficiency. It complements nucleic acid testing and offers critical insights into disease progression and severity. Serology has become an essential component of diagnostic strategies, particularly in cases where nucleic acid detection alone may not suffice. Continued advancements in serological testing will further enhance its diagnostic capabilities and contribute to improved patient care.
The outbreak of Covid-19, caused by the SARS-CoV-2 virus, resulted in a severe respiratory illness that posed a significant threat to public health. Serological testing played a crucial role in diagnosing SARS-CoV-2 infection, having been instrumental in studying the virus’s epidemiology, conducting serosurveillance, and advancing vaccine research and development.
Multiplexed immunoassay technologies offer a notable advantage as they enable the simultaneous detection of multiple analytes from a single sample. Among these technologies, xMAP stands out as a powerful multiplex analysis platform capable of measuring up to 500 analytes simultaneously from the same sample. This technology has proven invaluable in investigating the immune response to various SARS-CoV-2 antigens and assessing host protein biomarker levels as prognostic indicators for Covid-19.
While only 50 percent of patients with SARS-CoV-2 infection had positive IgM and IgG in their serum within a week of symptom onset, antibody concentrations increased over time, reflecting disease severity and providing valuable diagnostic information. Studies have demonstrated that IgM and IgG levels were significantly higher in severe Covid-19 cases, compared to mild-to-moderate cases. Some serological tests also target IgA, which shows higher concentrations in the early stages of infection. Combined detection of multiple antibodies has been proposed to enhance diagnostic accuracy.
Researchers also developed a serological nano-biosensor that could quickly detect and quantify SARS-CoV-2 antibodies in blood serum. The biosensor, based on plasmonic technology, provides results in less than 15 minutes without the need for sample pre-treatment. It has shown high sensitivity and specificity in detecting Covid-19 antibodies, outperforming existing diagnostic methods.
Scientists from the Singapore-MIT Alliance for Research and Technology (SMART) and Nanyang Technological University (NTU Singapore) developed a quick test kit that can assess a person’s immunity to Covid-19 and its variants, based on the detection of antibodies in a blood sample. Unlike antigen-based tests, this serology test measures antibodies produced by the patient, providing results in just 10 minutes, compared to the 24 to 72 hours needed for conventional laboratory testing. The test kit detects neutralizing antibodies against SARS-CoV-2 and its variants, including Delta and Omicron, and can be adapted for future variants and other diseases. With its low cost, speed, and accuracy of up to 93 percent, the test kit enables personalized vaccination strategies by determining the individual’s antibody levels and immune response.
Multiplex serological assays allow for the simultaneous detection of multiple antibodies or antigens in a single patient sample. These assays use microarray or bead-based technology, enabling high-throughput screening and providing comprehensive diagnostic information. The Luminex xMAP technology is a popular example of multiplex serological assays.
NGS techniques have been applied to serological testing, allowing the identification and characterization of complex antibody responses. NGS provides a detailed view of the antibody repertoire, aiding in the diagnosis of autoimmune disorders, infectious diseases, and cancer.
Point-of-care advancements. Advancements in serological testing have led to the development of portable and user-friendly point-of-care devices. These devices provide rapid results, enabling immediate diagnosis and treatment decisions at the patient’s bedside.
Inflammatory biomarkers in Covid-19
 Jason Armstrong
Jason Armstrong
Scientific Content Creator,
Randox Laboratories Ltd
Over the course of human history, few events have had as drastic an impact as the Covid-19 pandemic. According to the World Health Organization (WHO), as of July 12, 2023, the SARS-CoV-2 virus has claimed almost 7 million lives globally, and figures continue to rise.
While some who become infected are only subject to mild symptoms, many will develop a more severe form of the infection and are encumbered with a debilitating flu-like condition, often requiring days, if not weeks of bed rest. In a paper from June 2023, C reactive protein (CRP) concentrations were quantified, using an RX Imola, along with other biomarkers, in mild and severe Covid-19 patients to develop novel risk stratification methods for this potentially life-threatening viral infection.
In times of medical emergency, it is crucial to have an efficient and effective means of stratifying the risk to patients, and a process for suitably categorizing those into the least- and most-at-risk of severe complications or death. Due to the rate at which Covid-19 spread, unfortunately, the world lacked these mechanisms for SARS-CoV-2.
CRP is a non-specific, acute-phase protein, meaning its concentration is altered in response to inflammation. The acute respiratory distress syndrome, induced by SARS-CoV-2, is in part a result of the hyperinflammation caused by the virus. CRP is a well-characterized inflammatory biomarker and is, therefore, well-suited for identification and risk stratification in an emerging disease.
Paranga et al. studied a total of 13 biomarkers to determine which could accurately differentiate mild, moderate, and severe cases, and identify biomarkers which were good predictors of fatality.
This paper compares CRP to 12 other biomarkers including suPAR, sTREM-1, ferritin, MCP-1, and lactate dehydrogenase. Of these, it was discovered that CRP was clearly the most effective biomarker for differentiating mild from severe cases, with concentrations in those with severe infection being, on average, 45 percent higher than in those with mild symptoms.
Additionally, the authors discovered that CRP levels were not significantly affected by age, a factor known to affect the inflammation and immune responses, potentially providing a powerful and inclusive risk stratification tool.
Market dynamics
The global serological testing market in 2022 was USD 4.52 billion and is estimated to reach USD 7.6 billion by the end of 2030 with an annualized growth rate of 6.7 percent through 2023–2030.
The market has witnessed a notable shift away from the traditional ELISA method toward the increasing usage of chemiluminescence immunoassay (CLIA), primarily due to CLIA’s superior sensitivity and accuracy over ELISA. Moreover, the increased adoption of CLIA by expert professionals has driven the need for serological testing. Another contributing factor to the growth is the rise in antibody tests, which can be attributed to improvements in test accuracies and their acceptance by experts.
The growing prevalence of serological testing is generating a demand for consumables and reagents, which is propelling the market growth. There has been increasing incidence of TB in the recent years that has led to the growth of the serological testing. India’s TB incidence for the year 2021 is 210 per 100,000 population than the baseline year of 2015. The incidence was 256 per lakh of population in India and there has been an 18 percent deterioration. TB is the 13th leading cause of death worldwide and the second leading cause of infectious killer after Covid-19.
The major constraint of the market is the lack of reimbursement policies across the globe. High operating and maintenance costs also act as factors responsible for stalling growth. Lack of good-quality healthcare facilities in developing countries and higher diagnostic cost not afforded by numerous patients, mainly belonging to the lower economic groups. hamper market growth.
Regional insights. North America accounts for the major serological testing market. The reasons are many but the most important being well-established healthcare infrastructure. Rise in the number of geriatric populations is also a reason.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030 due to the increase in awareness, and rise in per capita healthcare expenditure, along with huge rate of infectious diseases in developing countries, such as India and China.
Indian market
Serological testing witnessed a negative demand shock across India amid the pandemic. Fortune Business Insights has pegged the decline at 15.2 percent in 2020. The market that had steadily been seeing an increase every year, fell from ₹1700 crore in 2020 to ₹1530 crore in 2021. The lockdown and the stringent guidelines imposed during the initial days of the Covid-19 outbreak posed extreme challenges. The healthcare organizations struggled due to the uncertainties regarding manpower. Furthermore, the supply chain disruptions, lack of availability, and the high price of raw materials affected the overall market. The timely availability of lab reagents was also one of the biggest concerns. Several lab reagents and kits belonged from international markets and, therefore, due to supply chain disruptions, there was a constraint in logistics, thus negatively affecting the India serological testing market growth.
Blood banks in India also witnessed a negative impact in terms of blood donations during the pandemic. Although the spread of the SARS-CoV-2 virus has not been detected through blood transfusion, anxiety and fear led to a significant decrease in the number of blood donations. Furthermore, despite taking preventive measures during blood donation campaigns, there was a negative impact on blood donations, affecting the blood supply and the blood transfusion process. Due to the lack of blood bag units’ availability, comparatively fewer serological tests were performed, thus reducing the requirement for the kits and reagents and negatively impacting the market. Also, cancellations and postponement of surgical procedures led to a decline in pre-operative serology testing. The serological screening tests for infectious diseases like HIV, HCV, and HBV also declined due to the ongoing pandemic. Patients avoided mandatory screening tests due to the fear of the SARS-CoV-2 virus.
However, it is now back on track from 2022, poised at a CAGR of 16.1 percent. The sudden rise in CAGR is attributable to this market’s growth and demand returning to pre-pandemic levels once the pandemic is over.
There is huge scarcity of blood during an emergency or elective procedures that the patients face. However, with lot of awareness created, donations are coming forth, providing the requisite impetus for serological tests in the country.
Major players are involved in the development and launch of novel products to increase their market presence. Some of the prominent players operating in serological testing include Randox Laboratories Ltd., Thermo Fisher Scientific, Inc., Beckman Coulter, Inc., Cellex Inc., Advanced Diagnostics, Inc., Eurofins Scientific, Abbott, Becton, Dickinson and Company (BD), Quest Diagnostics, F. Hoffmann La-Roche Ltd., and BioMedomics Inc.
Serology testing is done by numerous methods, such as enzyme-linked immunosorbent assays (ELISAs), hemagglutinin-inhibition tests, flocculation tests, chemiluminescence immunoassays, and neutralization tests.
Outlook
It may be safely stated that detection methods, such as sequencing, will not replace serology (e.g., lateral flow assays) anytime soon. Molecular testing is complementary to, but not a replacement for, classical testing methods. Serology testing market is on a growth trajectory worldwide, at least till 2030.












