MB Stories
Unlocking the power of immunochemistry

Immunochemistry stands as a beacon of innovation, promising breakthroughs in diagnostics, treatment, and disease management. From precision diagnostics to personalized therapeutics, it offers a multifaceted approach to addressing the complex challenges of today’s medical world.
In the ever-evolving landscape of healthcare, the fusion of cutting-edge science and innovative technology continues to reshape the paradigms of medical diagnosis and treatment. Among the most promising frontiers lies immunochemistry, a discipline harnessing the intricate interplay between the immune system and chemical analysis to propel healthcare into a realm of unprecedented precision and efficacy. Through a symphony of research, expertise, and technological prowess, immunochemistry offers unparalleled insights and solutions to address the most pressing challenges in healthcare.
Immunochemistry analyzers are becoming popular as they are effective tools to diagnose various diseases including cancer, hepatitis, illegal drugs, fertility problems, infectious diseases, and chronic diseases.
From point-of-care (POC) diagnostics to high-throughput screening platforms, immunochemical assays form the cornerstone of modern healthcare, empowering clinicians with the tools needed to make timely and informed decisions that can mean the difference between life and death.
Indian market dynamics
The Indian immunochemistry analyzers and reagents market in 2023 is estimated at ₹3245 crore. Reagents continue to dominate the segment at ₹2975 crore, with instruments at ₹270 crore. The instruments and reagents are poised at a 7.5-percent CAGR.
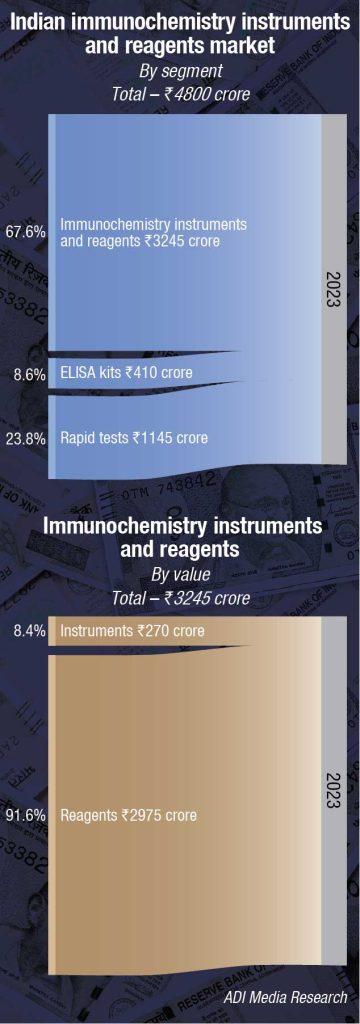
Additionally, the rapid tests’ market is estimated at ₹1145 crore and ELISA kits at ₹410 crore in 2023. This year, while the number of tests increased, realization per test was much lower.
Chemiluminescence immunoassays (CLIA) that constitute almost 80 percent of the immuno diagnostic market are indispensable tools in Indian medical landscape, offering a broad spectrum of applications encompassing infectious disease testing and cancer biomarker analysis. Consequently, they play a pivotal role in enhancing healthcare delivery, facilitating disease management, and advancing public health initiatives in the country. Against this backdrop, India’s CLIA analyzers market is expected to grow at a CAGR of around 6 percent through 2033, forecasts GlobalData.
|
Leading players-Indian immunochemistry instruments and |
|
| Tier 1 | Roche |
| Tier 2 | OCD, Siemens and Abbott |
| Tier 3 | Beckman Coulter and bioMérieux |
| Tier 4 | Snibe, Mindray, Diasorin, Tosho, Matrix, CPC and Agappe-Maccura, Randox, Beacon and Transasia* |
| Others | Autobio Diag, and Bio Rad* |
|
*Elisa and Rapid only ADI Media Research |
|
Despite the limitations, including technology dependency, limited R&D investment, and market competition, India’s cost-effective manufacturing capabilities stemming from lower labor and operational expenses, underscore its appeal as a manufacturing hub.
Recently, Agappe Diagnostics Ltd. have entered into an agreement with Fujirebio Inc., Japan, of contract development and manufacturing organization (CDMO) partnership for cartridge-based CLIA system reagents manufacturing project for the immunology equipment Mispa i60 and Mispa i121. While Fujirebio will supply equipment and raw materials, Agappe will develop and manufacture dedicated reagents, meeting regulatory standards. By combining the mutually complementary strengths of both companies, Agappe and Fujirebio can deliver high-quality CLIA solutions with cost competitiveness, contributing to healthcare advancement in India. Agappe and Fujirebio are set to launch the analyzers and reagents in June 2024 after significant progress, including signing a supply and license agreement in March 2023. Notably, these analyzers and reagents will be marketed under Agappe’s brand, marking Agappe as the first Indian company to offer a complete chemiluminescence solution with locally manufactured reagents.
Global market dynamics
The market for immunochemistry is experiencing robust growth, fuelled by increasing demand and rapid technological advancements.
The global immunochemistry market was valued USD 3.34 billion in 2023 and is expected to reach USD 4.56 billion by 2030, growing at a CAGR of 7.1 percent from 2024 to 2030, according to Verified Market Research.
The growth of the reagents and kits is increasing due to high consumption and their repeated purchases along with the increasing number of immunoassay tests.
Some of the key drivers have a big impact on how immunochemistry is in demand and adopted in different sectors. For the early diagnosis and monitoring of chronic diseases, including cancer, cardiovascular disease, and autoimmune disorders, growing prevalence of age-related disorders demand an ongoing improvement in the accuracy and efficiency of diagnostic testing, and development of multiplex and high-sensitivity immunoassays.
Immunochemistry techniques, ELISA and CLIA, have emerged as powerful tools for biomarker detection and analysis. These techniques enable the quantification of specific proteins and molecules in biological samples, providing clinicians with valuable information for patient care. Additionally, the development of multiplex immunoassays allows for the simultaneous detection of multiple analytes from a single sample, enhancing diagnostic efficiency and reducing the need for multiple tests.
The incorporation of robotics and automation into immunochemistry technologies not only enhances test accuracy and reduces turnaround times but also significantly elevates overall laboratory productivity. The exceptional specificity and sensitivity inherent in immunoassays render them as invaluable tools for precise disease monitoring and diagnosis, consequently driving the market’s expansion.
Emerging trends in immunochemistry
 Roshan D Raul
Roshan D Raul
Asst Product Manager-Immunology,
Transasia Bio-Medicals Ltd.
With advancements in technology and a growing emphasis on precision medicine, the field of immunochemistry continues to evolve rapidly, shaping the landscape of healthcare diagnostics. Continuous innovation in CLIA reagents and instrumentation has led to significant enhancements in assay performance. Manufacturers are investing in novel chemistries, signal amplification techniques, and optimized protocols to achieve unparalleled sensitivity and specificity. This allows for the detection of low-abundance analytes with exceptional accuracy, even in complex biological matrices. Rising incident rate of cancer is foreseen to be the key driver in immunochemistry. Availability of advanced diagnostic technologies is contributing in early diagnosis and also in monitoring the prognosis of patients.
Automation and integration. One of the prominent trends in immunochemistry instruments is the integration of automation features. Automated immunoassay systems streamline workflows, reduce manual errors, and enhance efficiency in diagnostic laboratories. These systems offer high throughput capabilities, enabling faster turnaround times and increased sample processing capacity. Integration with laboratory information systems (LIS) facilitates seamless data management and result interpretation, contributing to improved clinical decision-making.
Multiplex assays. Multiplex immunoassays enable the simultaneous detection of multiple analytes within a single sample, providing comprehensive diagnostic insights. This capability is particularly valuable in the diagnosis and monitoring of complex diseases, such as cancer, autoimmune disorders, and infectious diseases. Multiplex immunoassay platforms utilize advanced technologies, such as microarray-based assays and bead-based assays, to enhance sensitivity, specificity, and throughput. The ability to measure multiple biomarkers simultaneously enhances diagnostic accuracy and accelerates disease characterization, paving the way for personalized treatment approaches.
Enhanced sensitivity and specificity. Continuous innovation in immunochemistry reagents is driving improvements in assay sensitivity and specificity. Novel conjugation methods, signal amplification techniques, and optimized assay protocols contribute to enhanced analytical performance, enabling the detection of low-abundance analytes with exceptional precision. These advancements are particularly beneficial in the early detection of disease biomarkers, prognostic evaluation, and therapeutic monitoring, facilitating timely interventions and improved patient outcomes.
A notable trend is the rise of multiplex immunoassays, allowing for the simultaneous detection of multiple analytes that enhance the comprehensiveness of diagnostic results and streamline testing procedures. The adoption of AI for data analysis in immunoassays contributes to improved accuracy and efficiency in interpreting test results.
Challenges. Growth is projected to be limited by the cost of immunoassay tests, particularly sophisticated and specialized assays. Certain immunoassay techniques are complicated and require specialized equipment and personnel training, which can make implementation and execution difficult. Certain immunoassay tests, especially those sent to central laboratories, may have lengthier turnaround times, which could cause delays in the determination of the diagnosis and course of therapy. In addition, growth is hampered by delayed product approvals, human shortage, and financial constraints, particularly in developing countries.
However, the global immunochemistry market is expected to be showcasing a healthy growth rate in coming years. This may be attributed to the multiple advantages, from high sensitivity and versatility to rapid results and broad applications, the modality offers.
Region insights. North America dominated the market with a leading share exceeding 38.5 percent, equating to a market value of USD 12.3 billion in 2023. This is attributed to several factors, including substantial investments in biomedical research by organizations like the NIH, which are expanding the utilization of immunoassays in academic laboratories. Additionally, the presence of key industry players ensures easy accessibility to the latest immunoassay products. Furthermore, the increasing prevalence of chronic and infectious diseases in the region contributes to the growing demand for immunoassay technologies.
In the Asia-Pacific region, the market is estimated at the highest CAGR owing to rapid advancements in biotechnology sector and factors like growing ageing population, large patient population, and presence of emerging economies are expected to create growth opportunities for the market players.
Latest trends in immunochemistry equipment and reagents
 Thomas John
Thomas John
Managing Director,
Agappe Diagnostics Ltd
In the realm of healthcare and biomedical sciences, immunochemistry plays a pivotal role in diagnostics, therapeutics, blood protein analysis, and research. Immunochemistry instruments and reagents. Immunochemistry analyzers have proved to be very effective tools to diagnose the diabetes, inflammation, fertility issues, tumors, bone metabolism disorders, and other kinds of infectious and chronic diseases. Among the key methodologies within immunochemistry, nephelometry stands out for its significance in diagnostics and research.
Technological advancements
-
Multiplexing and high-throughput platforms. Enable simultaneous measurement of multiple analytes, speeding up research and clinical workflows.
-
Automation and robotics. Streamline workflows, enhance reproducibility, and improve efficiency with reduced manual labor.
-
Miniaturization and microfluidics. Develop compact devices for immunoassays, offering precise control and faster assay times with enhanced sensitivity.
-
Increased sensitivity and specificity. Technological improvements in platforms like CLIA and nephelometry offer more accurate detection at lower concentrations.
Emerging trends
-
Point-of-care testing. Fuels the development of portable immunoassays for rapid, real-time biomarker detection in various settings.
-
Innovations in reagent development. Continual reagent innovation boosts assay performance, enabling reliable biomarker detection.
-
Nanotechnology and bioconjugation strategies. Enhance assay sensitivity, specificity, and stability, promising new diagnostics and therapeutic avenues.
Agappe is striving to provide the advanced techniques in immunochemistry instruments and reagents with the advanced nephelometry system, such as Mispa i2 and Mispa i3, offering more than 25 ready-to-use LEIT reagents that can also perform with any automated or semi-automated nephelometry analyzer, including key tests like Vitamin D and special parameters like cystatin C, KPPA, LAMDA light, immunoglobulins IgA, IgE, IgG, IgM, Lp(a), APO-A, APO-B, C3, C4, ceruloplasmin, D-dimer ferritin, etc. Agappe offers very cost-effective, convenient pack sizes that can suit low-throughput labs as well as higher-turnover labs, with pre-calibrated reagents.
POC immune profiling technologies
Immunochemistry market is predicted to grow further with the increasing development of point-of-care (POC) testing for quick and decentralized diagnostics, high demand due to the push toward personalized medicine and targeted medicines.
Point-of-Care immune profiling technologies emerge as transformative tools in the battle against diseases, particularly cancer. Harnessing the power of innovative approaches, such as microfluidics-based cell sorting chips and ultrasensitive immunoassays, these technologies offer a paradigm shift in how we understand and combat illness. By enabling clinicians to monitor the immune response of cancer patients in real time, POC immune profiling holds the promise of revolutionizing treatment strategies, leading to more personalized and effective therapies.
Traditionally, immune profiling using flow cytometry has been confined to specialized laboratories due to the high cost, large size, and complexity of equipment. However, the advent of novel cell sorter chip technology marks a significant breakthrough, bringing immune monitoring directly to clinical practice. By miniaturizing the process onto a portable chip, about the size of a lunchbox, researchers at India-Middle East-Europe Economic Corridor (IMEC) have paved the way for routine immune profiling in settings, such as oncologists’ offices or daycare centers. This advancement not only streamlines the monitoring process but also eliminates the need for time-consuming sample transportation and specialized conditions, making it accessible to a wider patient population.
What sets POC analyzers apart is their portability and affordability, democratizing access to early disease detection. These devices empower healthcare providers to intervene proactively, even in resource-limited settings, thereby mitigating the impact of diseases and improving patient outcomes. As we navigate the complexities of modern healthcare, the emergence of these innovative technologies heralds a new era of personalized medicine.
How immunochemistry technology (ICT) has inspired the improvement of healthcare
 Prof Praveen Sharma
Prof Praveen Sharma
Scientific Consultant,
Snibe Diagnostics
The development. As is well known, for the vast majority of diseases, serological testing remains the most commonly used methodology in clinical diagnostics. Among these, immunoassays, biochemistry, and hematological assays are the main components. Over the last few decades, prominent medical manufacturers worldwide have actively researched and advanced immunoassays due to their convenient and comprehensive benefits. This has set the groundwork for the swift progress and evolution of immunoassay technology.
Reviewed at the development process of immunoassay, from the initial fluorescent antibody technique to the RIA, afterwards the widely used ELISA was discussed. Through the research and development of past decades, now the most advanced and accurate CLIA is the latest technology of immunoassay. Compared with ELISA, CLIA is less susceptible to the interference of other substances in the environment, which can make the test results more reliable.
The application. Having experienced the pandemic, individuals are now placing greater emphasis on their health. Currently, immunological testing is extensively utilized. Whether it pertains to infectious diseases, tumors, or autoimmune conditions, immunological testing and the accompanying automated analyzer equipment have proved immensely beneficial for medical laboratories and research institutions, particularly in densely populated regions.
The future. Even though immunoassay can deal with most of detections, but sometimes it still shows some systematic error when detecting small-molecule sample, such as estradiol, aldosterone and 25-OH vitamin D. Small molecules are not amenable to traditional sandwich immunoassays due to their difficulty of simultaneous recognition by two antibodies. For this reason, the major IVD companies are heavily investing in the small molecule sandwich detection (SMSD) technology. SMSD is now using a novel technology of small molecule detection and the small molecules will be captured by magnetic microbeads coated with antibodies, forming immunology complexes. Then, another key marker antibody will combine with the complexes to create sandwich complexes. This novel technology is highly consistent with LC-MS method, especially in low level, which ensures the accuracy of the low-concentration test.
As the latest techniques advance and advanced AI is integrated, precise measurement and conversion of the intricate data generated by analysis is now more accurate and efficient. These advancements are set to become an essential and revolutionary tool for medical diagnosis.
Compact immunoassay analyzers for low- to mid-volume labs
In response to the pressing operational challenges faced by clinical laboratories, innovative solutions in the form of compact immunoassay analyzers have emerged, catering specifically to low- and mid-volume labs. These advanced systems offer streamlined workflows and enhanced efficiency, empowering labs to manage increased sample volumes and staff shortages effectively. With features like random access sampling and micro-volume aspiration, these analyzers ensure predictable turnaround times while maximizing uptime through automated maintenance scheduling.
They prioritize safety and security, with robust measures, such as user authentication and audit trails. Meanwhile, the automated mono-test chemiluminescence immunoassay (CLIA) analyzers further bolster the capabilities of smaller labs. These systems, characterized by their compact design and high sensitivity, deliver accurate testing results across a wide range of parameters, from cardiac markers to tumor markers. Offering rapid turnaround times and reliable performance, these analyzers represent a significant advancement in laboratory diagnostics, ensuring timely and informed decisions for patient care.
Patient-centric, smart quality controls for immunoassays
 Jason Armstrong
Jason Armstrong
Chief Scientific Content Creator,
Randox Laboratories Ltd
In 2022, an updated version of ISO15189 was released, placing an emphasis on risk management with the aim of mitigating risk to patients. This updated document means that rigorous quality control (QC) procedures are more important than ever.
ISO15189:2022 cites the use of third-party controls with commutable matrices manufactured to provide concentrations close to clinical decision limits, among others, as crucial considerations. ISO15189:2022 also highlights the importance of identifying and minimizing errors in the pre-analytical process. Load & Go or Smart controls are becoming increasingly popular in laboratories around the world to realize this objective.
Smart controls are designed to optimize laboratory workflows, allowing laboratorians to load the control onto an instrument where it can remain until its expiry date, bringing several advantages to laboratories who run immunoassays.
The first is the minimization of human error and other pre-analytical errors. As these controls are ready-to-go out of the box, there is no chance of reconstitution errors, which can result in deviations from the target values and contamination, which could lead to problematic cross-reactions. Smart QCs reduce the risk of stability issues resulting from aliquoting or the repetitive opening of vials, and eliminate the possibility of mislabelled controls, while freeing up more cold-storage space.
Smart controls also offer the possibility of improvements in other areas of the laboratory. The reduced preparation required for these controls allows laboratories to use this time improving other elements of their QC practices, such as QC analysis and process improvement. Fewer steps in the QC process not only means time saved in the process itself, but also less paperwork for the laboratory staff, further freeing up time for more useful practices. Workflow efficiency can also be improved through consolidation of analytes. Smart controls that effectively consolidate many analytes, while maintaining suitable stability, reduce the number of controls required.
Immunoassay smart QCs provide laboratories with an effective QC solution, which aids in the optimization of workflows and the reduction of test turnaround times and the risk of human error throughout the QC process. However, if considering a smart QC for your laboratory, it is important to remember the other factors too, which make a good QC including matrix, stability, and clinically relevant concentrations.
Implications of immunochemistry in healthcare
Recent advancements in immunoassay analyzers have paved the way for more precise and quantitative detection methods, revolutionizing diagnostic capabilities in clinical laboratories. Equipped with innovative features like random access sampling and micro-volume aspiration, these analyzers offer increased throughput rates and turnaround times, addressing the growing demands on healthcare systems worldwide.
Moreover, the integration of remote service and diagnostic solutions ensures proactive system servicing and troubleshooting, further enhancing operational performance and minimizing workflow disruptions.
Biomarkers play a pivotal role in disease diagnosis, prognosis, and treatment monitoring. Researchers are continually exploring new biomarkers that can provide early detection of cancer, infectious diseases, and neurodegenerative disorders, facilitating early intervention and improving patient outcomes by enabling timely and targeted treatments. The validation of novel biomarkers requires rigorous testing and validation protocols to ensure their reliability and clinical utility.
In addition to disease diagnosis and monitoring, immunochemistry techniques are increasingly being utilized in therapeutic drug monitoring and pharmacokinetic studies. These techniques enable the quantification of drug levels in patient samples, ensuring optimal drug dosing and efficacy while minimizing the risk of adverse drug reactions.
Furthermore, immunochemistry has significant implications for the development of next-generation therapeutics, including immunotherapies and targeted therapies. These therapies harness the power of the immune system to combat diseases, such as cancer and autoimmune disorders, offering promising new treatment options for patients.
With advancements in technology, such as the utilization of semiconductor quantum dots (QDs) in fluorescence immunoassays, researchers are enhancing the sensitivity and multiplexing capabilities of immunoassay platforms.
Technological advancements and emerging trends in immunochemistry instruments and reagents
 Sathish Kumar
Sathish Kumar
Deputy Product Manager – CLIA,
Mindray India
The term automation refers to the execution of processes with only minimal involvement of an analyst.
Today, we are confronted with a new world of automation – a complex integration of robotics, liquid handling, and numerous other technologies with fundamental purpose of saving time and improving the performance through elimination of human error and reduced risk of cross contamination.
The large-scale clinical laboratories are under high pressure to improve their report quality, to reduce costs, to increase revenues, to grow capacity, and to deliver results faster against their common challenges of growing sample workload, shortage of skilled manpower, regulatory requirements, and instrument maintenance.
The integration of chemistry and immunoassay onto one system has captured the direct attention of laboratories everywhere to overcome the above challenges.
Consolidating instruments not only reduces the hardware footprint in the laboratory but also offers operational benefits, such as reduction in the periodic maintenance, single-tube processing, increased sample-loading capacity, broader test menu, significant walkaway capacity, and effective planning on the reagents and consumables inventory.
Mindray provides individual chemistry and immunoassay analyzer with different throughputs, which suits low- to medium- to high-workload laboratories. We also have chemistry and immunoassay integrated SAL series, which will be a one-stop solution for the core laboratories.
Mindray serum automation line (SAL) SAL-6000 and SAL-9000 is a high-performance integrated system, connecting chemistry analyzer seamlessly with the chemiluminescence immunoassay analyzer. With patented automatic serum analysis design, SAL series not only helps you optimize the management of lab space and manpower, but also offers highly reliable test results. The immunoassay platform in Mindray is equipped with most advanced chemiluminescence technology which have very high sensitivity, specificity, and wide assay menu.
Mindray has been providing scalable, automated solutions designed to optimize workflow, and maximize lab efficiency without sacrificing quality. With Mindray’s reliable solutions, high-volume laboratories can address their challenges of growing sample workload, shortage in manpower, regulatory requirements, and instrument maintenance.
We at Mindray believe in continuous improvement, and contemplate the future of healthcare with innovative thinking and developing newer technologies for better patient care.
The integration of artificial intelligence (AI) and machine learning algorithms into immunochemistry platforms holds immense promise. These technologies analyze vast datasets generated by immunoassay analyzers, identifying patterns and correlations that may not be apparent to human observers. By leveraging AI-driven insights, clinicians can make more informed decisions regarding patient diagnosis, treatment selection, and prognosis prediction. Furthermore, AI-powered diagnostic platforms have the potential to streamline workflow processes, improve diagnostic accuracy, and reduce healthcare costs.
Additionally, the development of new sensors, based on QD-fluorescence-linked immunosorbent assays (FLISA), represents a burgeoning area of research, holding promise for future diagnostic applications. These advancements not only improve diagnostic accuracy but also facilitate high-throughput screening, allowing for efficient analysis of large sample volumes in clinical and research settings.
Integrated automation in IVD laboratories
 Sanjay Patwardhan
Sanjay Patwardhan
General Manager – Sales & Marketing,
Biogeny Diagnostics (A Beacon Group Company)
The era of automation in clinical chemistry arrived with the introduction of the autoanalyzer, using continuous flow analysis, and the robotics that automated the traditional manual steps. Successive generations of analyzers increased analytical speed, allowing the testing of high volumes of specimens and providing large assay menus. A diversity developed, with development of immunoassays using a variety of methodologies.
However, with in last decade, development of integrated systems greatly improved the analytical phase of laboratory testing. Further, automation was developed for pre-analytical procedures, and post-analytical procedures. All phases of testing were ultimately combined in total laboratory automation (TLA) through which all modules are physically linked by some track system, moving samples through the process from beginning-to-end. Another important facet of automation is informatics, including middleware, which interfaces the analyzer software to LIS and HIS. This software includes control of the overall operation of TLA and combines analytical results with patient demographic information to provide additional clinically useful information.
A bunch of technologies starting from simple colorimetry up to florescent particle immunoassay (FPIA) and chemiluminesence immunoassay (CLIA) are used in these integrated systems, making them more useful for the purpose for which they are designed. These analyzers use ready-to-use liquid reagents or at times reagents that are reconstituted by the laboratorian upon usage. Again, with development of technology some analyzers also use dry or damp reagents, which are reconstituted with diluent and mixed with specimen directly during testing. Devising this unit dose concept for reagent has helped greatly in avoiding problems like carry overs, residual reagent deterioration, or aerosol effect on reagents.
The integration of chemistry and immunoassay into one system has captured the attention of laboratories and many of them across the globe are showing increasing response for such modular systems. Laboratories are under tremendous pressure for quality, cost, revenue, and speed of output. Hence the drivers for development of modular systems would be quality, TAT, and economy in maintaining them.
Development of modular system has helped to free the laboratorian’s time and enhanced menu, upscalability, and improved overall performance are enough to cause revolution in IVD testing.
Immunofluorescence breakthroughs
Enzyme-linked immunosorbent assay (ELISA) has gained widespread acceptance in research institutes and enterprises due to its high sensitivity, excellent specificity, ease of operation, low cost, and minimal instrument requirements. It has found extensive use in detecting various conditions, such as HBVM, HIV, syphilis, prenatal and postnatal care, allergen screening, and SARS-CoV-2. ELISA encompasses antigen and antibody detection methods, including two-site sandwich enzyme immunoassays and indirect methods.
Meanwhile, the landscape of emerging technologies in spatial biology is rapidly evolving, with a growing number of high-plex platforms aimed at revealing biomarker expression in tissue sections. To address the need for highly resolved spatial multiplex biomarker detection, a fully automated sequential immunofluorescence method has been developed. This method, based on COMET™ technology, allows for lower turnaround times and well-preserved sample morphology over numerous cycles, enabling higher plex levels.
The technique facilitates a 40-plex assay within less than 24 hours, with the potential for further increasing plex levels by increasing iterations or using additional channels. The versatility of this method extends to various biological domains, including basic research and translational science, making it suitable for immune oncology and investigation of the tumor microenvironment (TME) composition and response to stimuli.
As spatial proteomics gains traction in the research community, the sequential immunofluorescence method is poised to become a staple in translational research, with promising prospects for clinical applications.
Digital immunoassay technique addresses the challenge of detecting low-abundance proteins crucial for clinical diagnostics. Digital immunoassays offer enhanced sensitivity and multiplexing capabilities, allowing for the detection of such proteins and improving diagnostic accuracy. These advancements are driven by innovations in microfluidic technology, optical instruments, and image-processing algorithms.
Multiplexing barriers, such as limited encoding capacity, are being addressed through advances in droplet microfluidics and antibody screening techniques. The future of digital immunoassays lies in their potential for streamlined and integrated workflows in clinical settings, beyond fundamental research. Clinical accessibility remains a challenge, with the need for integrated, portable devices suitable for on-site operations.
In the next decade, digital immunoassays are expected to focus on improving sensitivity and multiplexing in laboratory settings, while also developing simpler, cost-effective devices for clinical use in primary healthcare settings. These advancements hold promise for enhancing in vitro diagnosis and empowering researchers in the fight against diseases.
Outlook
In the next decade, the field of immunochemistry is poised for remarkable growth and innovation, reshaping the landscape of healthcare in profound ways. Rapid advancements in technology, including robotics, automation, and artificial intelligence, will drive increased accuracy, efficiency, and productivity in diagnostic testing. Point-of-care immune profiling technologies will enable real-time monitoring of immune responses, leading to more personalized and effective therapies, particularly in cancer treatment. Nanotechnology will revolutionize biosensing technologies, enhancing sensitivity and multiplexing capabilities for improved diagnostic accuracy.
Furthermore, the development of next-generation therapeutics, such as immunotherapies and targeted therapies, will offer promising new options for patients, supported by the continued validation and utilization of biomarkers in personalized medicine. Overall, immunochemistry is poised to transform healthcare over the next decade, with advancements driving improvements in patient outcomes and quality of life.












