Urinalysis Instruments and Reagents
Urinalysis is changing, courtesy a new generation of analyzers
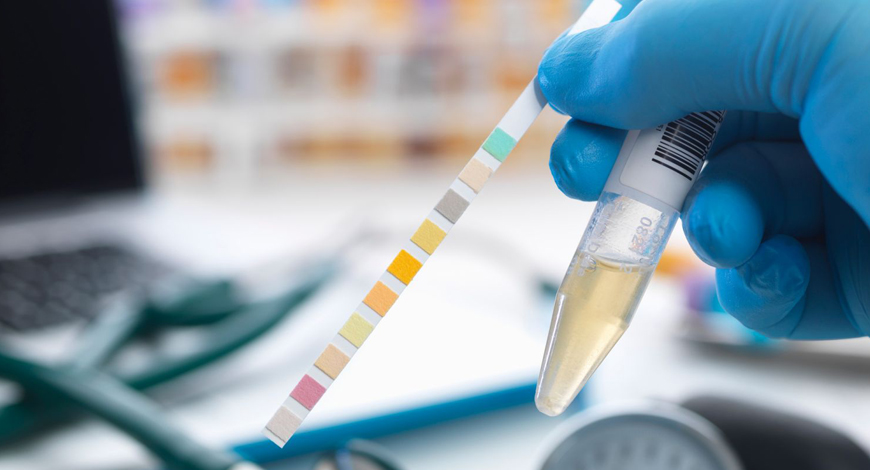
Over the past 25 years, new automated technologies and informatics have greatly reduced the labor intensity of urinalysis, and have created new technical possibilities.
Urinalysis is a major diagnostic screening test in the clinical laboratory, with an important role in diagnosing and monitoring nephrological and urological conditions. Until recently, microscopic urine sediment analysis was the most widely accepted urinalysis methodology. However, this time-consuming methodology is associated with extensive analytical errors. Over the past 25 years, new automated technologies and informatics have greatly reduced the labor intensity of urinalysis, and have created new technical possibilities.
New technological advances have paved the way for significant progress in automated urinalysis. Time and accuracy are the two key factors for diagnosis. In UTIs, urine dipsticks are very fast and easy to use, but the process lacks accuracy, whereas on the other hand, urine culture for antimicrobial susceptibility testing shows clinically reliable and accurate results, but it takes up to three days to give results. Many novel and improved diagnostic technologies and tools are introduced in the market, and some of them are already approved for clinical use and have helped significantly in increasing the accuracy and decreasing the time of the test; a good example would be nucleic acid tests and mass spectrometry. Some other technologies show promising future, such as the utilization of smartphone for urinalysis.
Recently, a new generation of analyzers that integrates and automates the two primary functions – chemistry and microscopic particle analysis – has been introduced. Automated microscopic and strip analyzers have been combined into fully automated workstations. These automated solutions are gaining popularity, which offer the phase-contrast images to classify the cells that are difficult to recognize. Microscopic images can be viewed in three different optical modes – bright field, phase contrast, and composite, a synthetic one. With the help of the stored images, the lab technician can use the system for new personnel training. With the phase-contrast option, particles like isomorphic, dysmorphic, and acanthocytes can be easily identified.
MALDI-TOF mass spectrometry (MS) has been recently introduced in routine clinical microbiology laboratories. As the time needed for culture continues to hinder decision-making and the laboratory workflow, direct-from-sample characterization of the bacterial load constitutes a major advance. Several studies have explored direct analysis of urine samples, using MALDI-TOF MS, thereby eliminating the time lag needed for pathogen identification. This technique has been suggested as a fast and reliable method for bacterial identification. Initial studies, combining urinary tract screening methods with direct application of MALDI-TOF MS in bacteriuria-positive samples, have demonstrated direct pathogen-identification sensitivity ranging from 67 percent to 86 percent. These results mirror those of similar studies showing successful pathogen identification from pathogen-positive blood cultures.
It is unclear whether MALDI-TOF MS can meet the demands of UTI diagnosis, given the need for screening to improve the yield of positive samples. For direct analysis of urine, initial sample preparation steps are necessary to remove cellular debris, WBCs, and mucus, and to collect bacteria. In its current iteration, analysis of MALDI-TOF MS results is hampered by poly-microbial samples. Up to 77 percent of the catheter-associated UTIs are poly-microbial; therefore, improved algorithms for interpreting the spectra of bacterial combinations are needed for direct-from-urine testing of these samples. Additionally, the technique does not provide reliable information on antimicrobial susceptibility for frequently used antibiotics in UTI treatment. Indirect approaches for antimicrobial susceptibility testing are under development, and include the measurement of bacterial metabolic byproducts in the presence of antibiotics to assess susceptibility.
Urinary flow cytometry and UTIs. Urine culture is considered the gold standard for UTI diagnosis. It can determine the level of bacteriuria and antimicrobial susceptibility. However, there is no standardized bacterial count, indicating significant bacteriuria, applicable for all types of UTIs. Scientific evidence supporting current urine culture guidelines is often incomplete, and in some cases, the guidelines do not indicate a clear choice. Because of the high percentage of negative results, there is a need for an efficient screening method, reducing the number of unnecessary culture tests. Several methods for screening-out culture-negative samples have been developed, including dipstick chemical tests (nitrite, leukocyte esterase, urinary protein, and urinary hemoglobin) and manual or automatic microscopic examination of urine sediment (detection of particles, WBCs, and microorganisms). Although these screening methods are primarily used in general practice and microbiology laboratories, they are subjective and time-consuming and demonstrate poor sensitivity and negative predictive value.
Many authors have reported using flow cytometry to detect bacteria and WBCs in urinary samples. Flow cytometry can reduce the number of samples cultured, with a substantial decrease in workload, time, and costs, especially in clinical laboratories. Using flow cytometry, negative results could be reported earlier, substantially reducing unnecessary empirical antibiotic prescriptions. The use of flow cytometry can reduce the number of urinary samples processed in the clinical laboratory by 28–60 percent. However, there is a wide variation in the applied cut-offs, as well as in the sensitivity and specificity of the obtained results in the literature. These variations are mainly owing to the spectrum of clinical conditions of the patient populations enrolled in these different studies. These differences can be attributed to the different definitions used to classify UTIs, which depend on the guidelines applied in a specific setting. Therefore, the applicability of flow cytometry to screen for negative urine samples strongly depends on population characteristics and the definition of a negative urine culture.
Although dry chemistry technology for urinary test strips has made limited progress, advances in electronic detection have considerably improved the analytical sensitivity of test strip readers over the years. Major improvement in the test strip technology has been made in recent years. Not only highly sensitive test strips are being introduced, but also now, one can find strips, which give quantitative results for urinary proteins. The financial aspect is also of great importance, especially in the third world and the developing countries; inexpensive test strips for various diagnostic reasons, such as the diagnosis of diabetes from urine sample are available. Test strip method also shows promising result in antibiotic susceptibility tests; if optimum diagnostic requirement is reached, it can reduce the test time significantly from 2 to 3 days to a few hours.
An interesting recent evolution is the use of smart phones for reading and interpreting urine test strip results. Mobile healthcare platforms have been proposed, combining a pocket-sized colorimetric reader and commercially available 10-parameter urinalysis paper strips, capable of sending data via a smartphone.
In view of the great need for the development of portable and cost-effective readers, pocket-sized colorimetric readers can be combined with dipsticks in a device that is able to transmit digital information via a smart phone, offering an integrated solution for detecting disease in areas with limited access to trained experts. Advances in microfluidics have enabled the development of new chip-based assays, which will alter the field of automated urinalysis in the near future. Alongside conventional urinalysis applications, integrated microfluidic chips have been described as a promising tool for measuring the concentration of bladder cancer cells in urine samples. Similarly, microfluidic paper analytical devices have been designed and fabricated for evaluating bacteria known to cause UTIs (Escherichia coli) and sexually transmitted diseases (Neisseria gonorrhoeae) in human urine samples.
Automated urinalysis has come a long way in the last two decades in terms of technology. Microscopy- and flow cytometry-based devices both produce therapeutically valuable results, and automated test strip reading adds to the usefulness. Integration of existing technologies may help to reduce turnaround times even more.
Meanwhile, laboratory consolidation has resulted in a reduction in the number of laboratories, increasing the physical distance between patient and laboratory; this development poses a significant pre-analytical difficulty. Despite advances in standardization, the majority of urinalysis errors occur outside of the analytical phase; pre-analytical processes are especially prone. Because analytical variation has been considerably minimized, more emphasis on the pre-analytical step is required.
Research Corner
Urine can reveal a lot about a person’s health. But physicians do not currently have a convenient or fast way of tracking the concentration of important compounds in their patients’ urine. Now, researchers reporting in ACS Applied Nano Materials have designed a flexible sensor that fits in a diaper, measures multiple components in urine and can share those results over Bluetooth to provide real-time bedside analyses for incontinent, elderly, or infant patients.
The concentration of certain compounds in urine can provide information about many different conditions, including kidney disease, urinary tract infections, and electrolyte deficiencies. Though many people with diabetes monitor their glucose levels with blood tests, glucose levels in their urine can also reveal spikes or dips. To analyze urine, however, physicians typically must order a urinalysis from a hospital lab, which takes time, or use paper test strips, which are not very sensitive. Neither system can deliver fast, bedside analyses. Some researchers have explored wearable devices to monitor health markers – like electrolyte and sugar content in sweat. So, Xi Xie, Hui-Jiun Chen, and colleagues wanted to design a similar type of wearable device that could accurately and sensitively measure the concentration of multiple health markers in urine and give real-time feedback to care providers.
The team first fabricated a flexible electrode array about the size of a US quarter. They included five different electrodes on the array that were designed to specifically detect potassium ions, sodium ions, hydrogen peroxide, uric acid, or glucose, which are biomarkers for various conditions. Then they connected the array to a circuit board that had a Bluetooth module and lithium-ion battery power source. When the array was exposed to urine samples from three volunteers, it performed as well as a commercial urine test system. Next, the researchers incorporated the array into a diaper and found that when urine was present, they could get readable signals for the biomarkers. However, they anticipate that in a real-world setting, where dry diapers become slowly saturated with urine, the electrode array would have to take multiple measurements to get stable readings. So, with optimization, this smart diaper could be a way to provide quick and painless urinalysis with wearable device technology, the researchers say.
The authors acknowledge funding from the National Key R&D Program of China; the National Natural Science Foundation of China; the China Postdoctoral Science Foundation; the Science and Technology Program of Guangzhou, China; the Guangdong Basic and Applied Basic Research Foundation; the Key Program of Sun Yat-Sen University; and the Pazhou Lab, Guangdong.












