Company News
Withings gets FDA nod for medical smartwatch ScanWatch
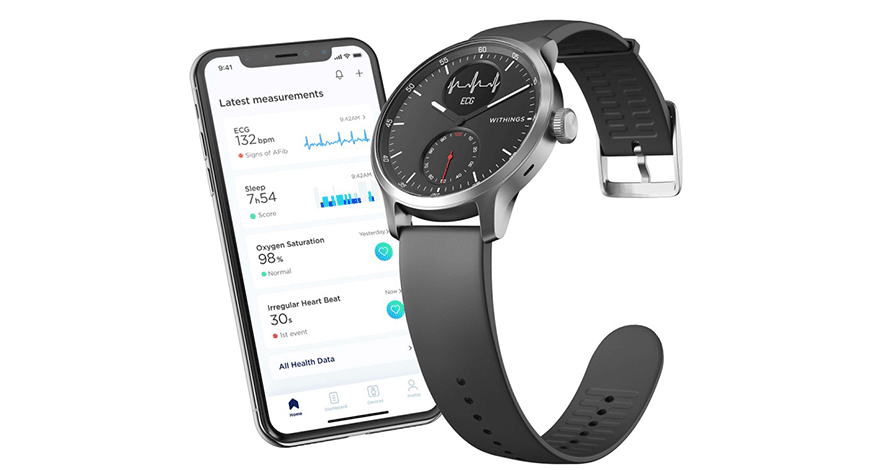
Withings said has received clearance from the Food and Drug Administration (FDA) for its medical-grade ScanWatch, a smartwatch that can detect heart rate problems.
The hybrid smartwatch and medical device can detect atrial fibrillation, a heart rate irregularity also known as “AFib,” through both electrocardiogram (ECG) and measurement of SpO2 levels from the wearer’s wrist. The latter is important in detecting whether someone has severe COVID symptoms.
Issy-les-Moulineaux, France-based Withings is one of the pioneers of connected health, and it can now start selling the device in North American in November. The device, already on sale in Europe and Australia, can make a big difference with its health measurements.
It looks like a classic wristwatch, but the device has sensors that can monitor heart rate, atrial fibrillation through ECG, breathing disturbances, blood oxygen levels through SpO2, sleep, and physical activity.
ScanWatch is the first wearable to simultaneously be cleared by the FDA to record ECG and SpO2 measurements. Priced from $279, it will be available from www.withings.com, Best Buy, and Amazon from early November.
“ScanWatch received three CES Innovation Awards when it was first announced in 2020, and we are now thrilled to bring it to the United States following robust FDA scrutiny,” said Mathieu Letombe, CEO of Withings, in a statement. “At Withings our core mission is to create beautiful devices people choose to use and wear every day so the medical data they provide can make meaningful impacts on their lives. ScanWatch has been clinically validated to detect AFib and can aid in the detection of breathing disturbances at night, that can be signs of sleep apnea. It is our most ambitious medical watch to date and has the potential to benefit millions of people.”
Developed with cardiologists and sleep experts, ScanWatch has been validated in two clinical studies. In Europe, it has been used in a study to monitor COVID patients remotely in German hospitals. It is designed with a stainless-steel case and durable sapphire glass watch face and features a large digital display as well as easy navigation through a newly created crown dial.
Additionally, ScanWatch is water-resistant up to five meters and features an exceptional battery life of up to 30 days.
In-depth cardiovascular health monitoring
AFib is a form of irregular heart rhythm that is often underdiagnosed, as it can be intermittent and easily missed if symptoms do not occur during infrequent doctors’ visits. ScanWatch can detect if a user has AFib thanks to its ability to take a medical-grade ECG on-demand. The device can monitor heart rate through its embedded PPG sensor, alerting the user to a potential heart event even if they don’t feel palpitations. In addition, when ScanWatch detects an irregular heartbeat through its heart rate sensor, it will prompt the user to record an ECG in just 30 seconds via the watch display.
ECG readings are displayed in the accompanying Withings Health Mate app where users can choose to send readings to their doctor or cardiologist.
Blood oxygen level and respiratory disorders
With FDA Clearance of its SpO2 functionality, ScanWatch can be used to monitor blood oxygen levels and can be used to help detect if someone is experiencing issues from respiratory disorders such as COPD or COVID. ScanWatch can also detect the presence of nighttime breathing disturbances (a sign of sleep apnea) with an exclusive algorithm that analyzes blood oxygen levels, heart rate, movement, and respiratory rate, all collected via the accelerometer and optical sensors.
In addition, ScanWatch provides sophisticated sleep monitoring and analysis of sleep patterns, including the length, depth, and quality of sleep, and can wake users up with a gentle vibration at the best time of their sleep cycle.
Activity and workout tracking
ScanWatch can monitor parameters such as steps, calories, elevation, and workout routes (via connected GPS) and can automatically recognize more than 30 daily activities such as walking, running, swimming, and cycling. In addition, it offers Fitness Level assessments through estimation of an indicator called VO2 Max, which measures the heart’s and muscles’ ability to convert oxygen into energy during physical exercise.
Like all Withings devices, ScanWatch connects with the free Health Mate app, which provides data and insights and can schedule activity reminders, set goals, and manage achievements. In addition, Health Mate can be paired with more than 100 third-party apps, including Apple Health, Google Fit, Strava, and MyFitnessPal. The 42 millimeter screen version is sold for $300, and it comes in a choice of black or white faces. Withings was founded in 2008, and its devices are used daily by millions of people.
The watch itself is the first analog watch with high-end design to combine medical-grade ECG and oxygen saturation through an SpO2 sensor with a rechargeable battery that lasts up to 30 days. Compared to an Apple Watch, the device specifically focuses on advanced health metrics, helping people identify and seek treatment for serious conditions. VentureBeat












