Life Sciences
PCR remains the mainstay of viral diagnosis
A rapid, accurate, low-cost, and easy-to-use test in the field could control the epidemic from becoming one. Unfortunately, despite all the advancements, that test still does not exist.
The 21st century has witnessed exponential growth in science and technology; however, sadly and ironically, it has succumbed to devastating virus infections: severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and now coronavirus (COVID-19).
 There are very few inventions that can compete with the importance of PCR over the past 100 years as it revolutionized biological and genetic research. At present, PCR and antibody testing are the dominant ways that global healthcare systems are testing citizens for COVID-19. Both techniques have their caveats, and as the crisis unfolds, researchers are looking into alternative ways to screen the deadly disease.
There are very few inventions that can compete with the importance of PCR over the past 100 years as it revolutionized biological and genetic research. At present, PCR and antibody testing are the dominant ways that global healthcare systems are testing citizens for COVID-19. Both techniques have their caveats, and as the crisis unfolds, researchers are looking into alternative ways to screen the deadly disease.
Over the course of the current COVID-19 crisis, the importance of reliable, accessible testing to screen for the disease has become increasingly apparent. The majority of tests for COVID-19 can be divided into PCR or serological tests. Both of these tests use different kinds of samples to search for different hallmarks of the SARS-CoV-2 virus – and neither of them is exactly perfect.
The RT-PCR test detects the genetic information of the virus, the RNA. That is only possible if the virus is there and someone is actively infected. PCR gives a good indication of who is infected. That is the true advantage of the current major diagnostic tests – one can break that transmission chain and get a clearer picture of what is happening.
By scaling PCR testing to screen vast swathes of nasopharyngeal swab samples from within a population, public health officials get a clearer picture of the spread of COVID-19.
PCR tests can be very labor-intensive, with several stages at which errors may occur between sampling and analysis. False-negatives can occur up to 30 percent of the time with different PCR tests, meaning they are more useful for confirming the presence of an infection than giving a patient the all-clear.
During the course of the outbreak, PCR testing has been refined from the initial testing procedures and with the addition of greater automation to reduce errors. As such, now it has an 80–85 percent specificity in detecting the virus.
 “Advancement in the field of molecular biology and the implementation of PCR testing for diagnostic purpose because of its accuracy and convenience and increase in research activities both in academics as well as pharmaceutical industries increases the demand of global thermal cycler market by the coming 2025.”
“Advancement in the field of molecular biology and the implementation of PCR testing for diagnostic purpose because of its accuracy and convenience and increase in research activities both in academics as well as pharmaceutical industries increases the demand of global thermal cycler market by the coming 2025.”
Dr Jyoti Upadhyay
Assistant Professor, School of Health Sciences, University of Petroleum and Energy Studies, Dehradun
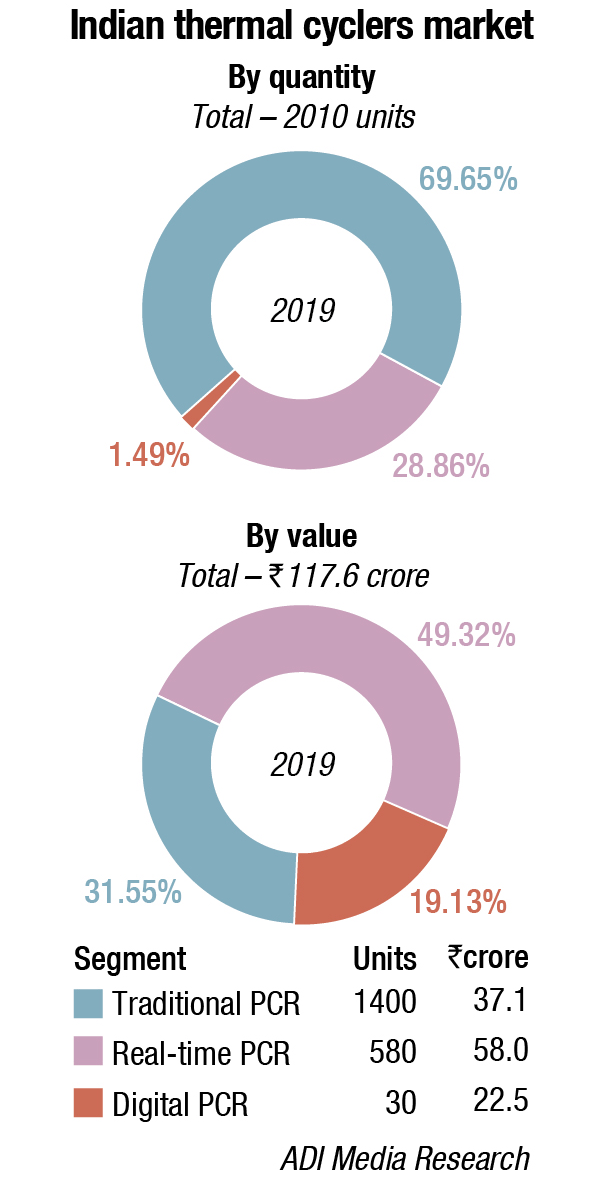
Indian market
Digital PCR (dPCR) is finding great acceptance in India. It is used for liquid biopsies in the areas of cancer diagnosis, monitoring, evaluation of treatment modalities, and testing for reoccurrence after remission. Diabetes and organ transplantation monitoring are discussed to demonstrate other emerging uses for liquid biopsy. It is a powerful technology that provides absolute quantification of nucleic acids with a high degree of sensitivity and precision.
In blood, the cfDNA, ctDNA, and CTCs of interest are present at low levels and found in a complex background of other components. Additionally, circulating DNA is highly fragmented, which further reduces the concentration of intact target sequence. Digital PCR has permitted the detection and quantification of low-abundance targets in shorter times without requiring large numbers of replicates. This has established dPCR as a tool of choice for liquid biopsies.
The multiplex PCR method is now being used for amplification of a genome of more than 30 kb. This protocol opens possibilities in the field of molecular virology, firstly because it can be easily applied to sequence and assemble other virus genomes. It is also of particular interest to explore over time the evolution of a virus genome during the infection process as one can follow which variants take over the others.
The medical fraternity has continued to find the reverse transcription-polymerase chain reaction (RT-PCR) as the gold standard frontline test for coronavirus (COVID-19) and the rapid antibody test is not able to replace it. MoHFW also issued a similar statement after the ICMR’s national taskforce had issued guidelines on testing strategy to all states, following a review of the worldwide testing methodology.
PoC into the spotlight
COVID-19, has brought the prospect of point-of-care (PoC) diagnostic tests into the spotlight. A rapid, accurate, low-cost, and easy-to-use test in the field could stop epidemics before they develop into full-blown pandemics. Unfortunately, despite all the advances, it still does not exist.
The COVID-19 global pandemic has greatly boosted the determination and motivation of researchers worldwide to speed up the development of POCT systems, as well as the number of companies prepared to fund such work. Some have adapted existing devices, while others have made new ones. More systems will emerge in the near future in the context of the COVID-19 pandemic, after which companies are likely to switch their interest to systems that are more viable from an economic perspective.
Future perspective
There are several techniques, such as NGS, where PCR plays an indispensable role and this role will be strengthened in the foreseeable future. PCR will play a major role in future molecular diagnostic techniques. We can envision the use of microfluidics performing parallel multiple sample analysis using handheld POC systems. Also, paper-based systems for PCR or LAMP will be in high demand for molecular diagnostics.
The miniaturization of PCR will continue with the development of new systems integrating sample preparation with the qPCR, moving toward truly portable sample-to-answer systems for POC applications. There will likely be more POC devices developed for SARS-CoV-2 and later converted into diagnostics for other RNA viruses.
The diagnosis systems would eventually be linked together forming an ‘Internet of Things’. The new systems would be standalone or based on a smart phone with an Android operating system. It has an advantage that the camera can be either controlled externally or internally and it can communicate with the temperature and illumination control via Bluetooth. Once the image is captured, the Android system could process it and send it to a control center via a wireless network.
We can also envision portable dPCR systems with fast turnaround for clinical diagnostics, forensic science, and environmental research, avoiding time-consuming sample transfer to large facilities that risks sample degradation.












